Abstract
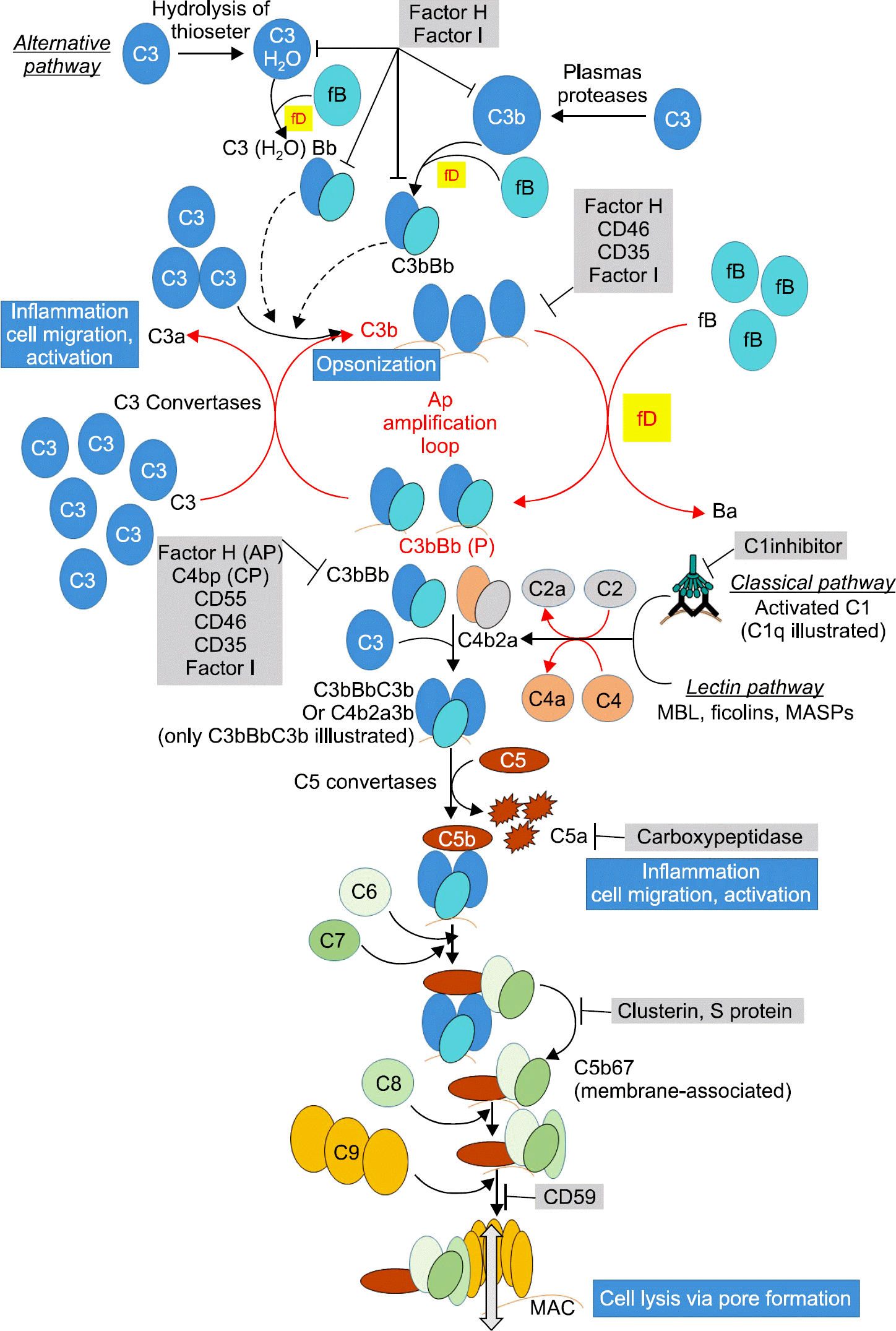
The complement system is a part of the innate immune system that potentiates the ability of antibodies and phagocytic cells to clear microbes and damaged cells. The complement system consists of a number of proteins circulating as inactive precursors. It is stimulated mainly by three pathways: the classical pathway, the alternative pathway, and the lectin pathway. There are many genetic polymorphisms in this system, which can over-activate the immune system. In this study, we collected the polymorphisms reported to over-activate complement cascades that affect the immune system and induce autoimmune diseases.
Go to : 
REFERENCES
1). Darbani B., Noeparvar S., Borg S. Deciphering Mineral Homeostasis in Barley Seed Transfer Cells at Transcriptional Level. PLoS One. 2015. 10:e0141398.

2). Budding K., van de Graaf EA., Kardol-Hoefnagel T., Broen JC1., Kwakkel-van Erp JM., Oudijk EJ, et al. A Promoter Polymorphism in the CD59 Complement Regulatory Protein Gene in Donor Lungs Correlates With a Higher Risk for Chronic Rejection After Lung Transplantation. Am J Transplant. 2016. 16:987–98.
3). Krych-Goldberg M., Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol Rev. 2001. 180:112–22.

4). Racila DM., Sontheimer CJ., Sheffield A., Wisnieski JJ., Racila E., Sontheimer RD. Homozygous single nucleotide polymorphism of the complement C1QA gene is associated with decreased levels of C1q in patients with subacute cutaneous lupus erythematosus. Lupus. 2003. 12:124–32.

5). Slingsby JH., Norsworthy P., Pearce G., Vaishnaw AK., Issler H., Morley BJ, et al. Homozygous hereditary C1q deficiency and systemic lupus erythematosus. A new family and the molecular basis of C1q deficiency in three families. Arthritis Rheum. 1996. 39:663–70.

6). McAdam RA., Goundis D., Reid KB. A homozygous point mutation results in a stop codon in the C1q B-chain of a C1q-deficient individual. Immunogenetics. 1988. 27:259–64.

7). Topaloglu R., Bakkaloglu A., Slingsby JH., Mihatsch MJ., Pascual M., Norsworthy P, et al. Molecular basis of hereditary C1q deficiency associated with SLE and IgA nephropathy in a Turkish family. Kidney Int. 1996. 50:635–42.

8). Westra HJ., Peters MJ., Esko T., Yaghootkar H., Schurmann C., Kettunen J, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013. 45:1238–43.

9). Bellemore SM., Nikoopour E., Schwartz JA., Krougly O., Lee-Chan E., Singh B. Preventative role of interleukin-17 producing regulatory T helper type 17 (Treg 17) cells in type 1 diabetes in non-obese diabetic mice. Clin Exp Immunol. 2015. 182:261–9.
10). Ma YB., Fu SY., Ma YH., Liu HL. Relationship between SERPING1 rs2511989 polymorphism and age-related macular degeneration risk: a meta-analysis. Mol Vis. 2014. 20:1434–42.
11). Ennis S., Jomary C., Mullins R., Cree A., Chen X., Macleod A, et al. Association between the SERPING1 gene and age-related macular degeneration: a two-stage case-control study. Lancet. 2008. 372:1828–34.

12). Xu YY., Zhi YX., Yin J., Wang LL., Wen LP., Gu JQ, et al. Mutational spectrum and geno-phenotype correlation in Chinese families with hereditary angioedema. Allergy. 2012. 67:1430–6.

13). Ricklin D., Hajishengallis G., Yang K., Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010. 11:785–97.

14). Maller J., George S., Purcell S., Fagerness J., Altshuler D., Daly MJ, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006. 38:1055–9.

15). Sturfelt G., Truedsson L. Complement and its breakdown products in SLE. Rheumatology (Oxford). 2005. 44:1227–32.

16). Johnson CA., Densen P., Hurford RK Jr., Colten HR., Wetsel RA. Type I human complement C2 deficiency. A 28-base pair gene deletion causes skipping of exon 6 during RNA splicing. J Biol Chem. 1992. 267:9347–53.

17). Wetsel RA., Kulics J., Lokki ML., Kiepiela P., Akama H., Johnson CA, et al. Type II human complement C2 deficiency. Allele-specific amino acid substitutions (Ser189 →Phe; Gly444 →Arg) cause impaired C2 secretion. J Biol Chem. 1996. 271:5824–31.
18). Chen HH., Tsai LJ., Lee KR., Chen YM., Hung WT., Chen DY. Genetic association of complement component 2 polymorphism with systemic lupus erythematosus. Tissue Antigens. 2015. 86:122–33.

19). Chen X., Kang X., Zhao K., Zhao C. C2 rs547154 polymorphism and polypoidal choroidal vasculopathy susceptibility: a meta-analysis. Sci Rep. 2015. 5:8709.

20). Zhan X., Larson DE., Wang C., Koboldt DC., Sergeev YV., Fulton RS, et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat Genet. 2013. 45:1375–9.
21). Frémeaux-Bacchi V., Miller EC., Liszewski MK., Strain L., Blouin J., Brown AL, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008. 112:4948–52.

22). Botto M., Fong KY., So AK., Koch C., Walport MJ. Molecular basis of polymorphism of human complement component C3. J Exp Med. 1990. 172:1011–7.
23). Giles JL., Choy E., van den Berq C., Morgan BP., Harris CL. Functional analysis of a complement polymorphism (rs17611) associated with rheumatoid arthritis. J Immunol. 2015. 194:3029–34.

24). Tremblay GM., Janelle MF., Bourbonnais Y. Anti-inflammatory activity of neutrophil elastase inhibitors. Curr Opin Investig Drugs. 2003. 4:556–65.
25). Xu D., Hou S., Jiang Y., Zhang J., Cao S., Zhang D, et al. Complement C5 Gene Confers Risk for Acute Anterior Uveitis. Invest Ophthalmol Vis Sci. 2015. 56:4954–60.

26). Liu B., Wei L., Meyerle C., Tuo J., Sen HN., Li Z, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-re-lated macular degeneration. J Transl Med. 2011. 9:1–12.

27). DiScipio RG., Gehring MR., Pdack ER., Kan CC., Hugli TE., Fey GH. Nucleotide sequence of cDNA and derived amino acid sequence of human complement component C9. Proc Natl Acad Sci U S A. 1984. 81:7298–302.

28). Wang Y., Xu S., Su Y., Ye B., Hua Z. Molecular characterization and expression analysis of complement component C9 gene in the whitespotted bambooshark, Chiloscyllium plagiosum. Fish Shellfish Immunol. 2013. 35:599–606.

29). Seddon JM., Yu Y., Miller EC., Reynolds R., Tan PL., Gowrisankar S, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013. 45:1366–70.

30). Nishiguchi KM., Yasuma TR., Tomida D., Nakamura M., Ishikawa K., Kikuchi M, et al. C9-R95X polymorphism in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012. 53:508–12.

31). Miura T., Goto S., Iguchi S., Shimada H., Ueno M., Nishi S, et al. Membranoproliferative pattern of glomerular injury associated with complement component 9 deficiency due to Arg95Stop mutation. Clin Exp Nephrol. 2011. 15:86–91.

32). Gold B., Merriam JE., Zernant J., Hancox LS., Taiber AJ., Gehrs K, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006. 38:458–62.

33). Goicoechea de Jorge E., Harris CL., Esparza-Gordillo J., Carreras L., Arranz EA., Garrido CA, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007. 104:240–5.
34). Alexander P., Gibson J., Cree AJ., Ennis S., Lotery AJ. Complement factor I and age-related macular degeneration. Mol Vis. 2014. 20:1253–7.
35). Kavanagh D., Yu Y., Schramm EC., Triebwasser M., Wagner EK., Raychaudhuri S, et al. Rare genetic variants in the CFI gene are associated with advanced age-relate macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015. 24:3861–70.
36). van de Ven JP., Nilsson SC., Tan PL., Buitendijk GH., Ristau T., Mohlin FC, et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat Genet. 2013. 45:813–7.

37). Bienaime F., Dragon-Durey MA., Regnier CH., Nilsson SC., Kwan WH., Blouin J, et al. Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 2010. 77:339–49.

38). Hakobyan S., Harris CL., Tortajada A., Goicochea de Jorge E., García-Layana A., Fernández-Robredo P, et al. Measurement of factor H variants in plasma using variant-specific monoclonal antibodies: application to assessing risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008. 49:1983–90.

40). Lauer N., Mihlan M., Hartmann A., Schlötzer-Schrehardt U., Keilhauer C., Scholl HP, et al. Complement regulation at necrotic cell lesions is impaired by the age-related macular degeneration-associated factor-H His402 risk variant. J Immunol. 2011. 187:4374–83.

41). Schmidt BZ., Fowler NL., Hidvegi T., Perlmutter DH., Colten HR. Disruption of disulfide bond is responsible for impaired secretion in human complement factor H deficiency. J Biol Chem. 1999. 274:11782–8.
42). Schmidt CQ., Slingsby FC., Richards A., Barlow PN. Production of biologically active complement factor H in therapeutically useful quantities. Protein Expr Purif. 2011. 76:254–63.

43). Rougier N., Kazatchkine MD., Rougier JP., Fremeaux-Bacchi V., Blouin J., Deschenes G, et al. Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol. 1998. 9:2318–26.

44). Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S, et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003. 111:1181–90.

45). Pérez-Caballero D., González-Rubio C., Gallardo ME., Vera M., López-Trascasa M., Rodríguez de Córdoba S, et al. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am J Hum Genet. 2001. 68:478–84.

46). Venables JP., Strain L., Routledge D., Bourn D., Powell HM., Warwicker P, et al. Atypical haemolytic uremic syndrome associated with a hybrid complement gene. PLoS Med. 2006. 3:e431.
47). Malik TH., Lavin PJ., Goicoechea de Jorge E., Vernon KA., Rose KL., Patel MP, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012. 23:1155–60.

48). Mihlan M., Hebecker M., Dahse HM., Hälbich S., Huber-Lang M., Dahse R, et al. Human complement factor H-related protein 4 binds and recruits native pentameric C-reactive protein to necrotic cells. Mol Immunol. 2009. 46:335–44.

49). Hebecker M., Józsi M. Factor H-related protein 4 activates complement by serving as a platform for the assembly of alternative pathway C3 convertase via its interaction with C3b protein. J Biol Chem. 2012. 287:19528–36.

50). Zhao J., Wu H., Khosravi M., Cui H., Qian X., Kelly JA, et al. Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 2011. 7:e1002079.
51). Liszewski MK., Atkinson JP. Complement regulator CD46: genetic variants and disease associations. Hum Genomics. 2015. 9:7.

52). Lee HK., Na HK., Lee JY., Chang JW., Yang WS., Kim SB, et al. A case of familial atypical hemolytic uremic syndrome associated with complement factor H mutation in adults. Korean J Nephrol. 2009. 28:259–64. (이현기, 나희경, 이지영, 장재원, 양원석, 김순배, 등. 성인에서 발생한 보체 H 인자 돌연변 이에 의한 가족성 비전형적 용혈성 요독 증후군 1예. 대한신장학 회지 2009;28: 259-64.).
53). Caprioli J., Noris M., Brioschi S., Pianetti G., Castelletti F., Bettinaglio P, et al. Genetics of HUS: the implact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006. 108:1267–79.
54). Richards A., Kemp EJ., Liszewski MK., Goodship JA., Lampe AK., Decorte R, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2003. 100:12966–71.

55). Heckmann JM., Uwimpuhwe H., Ballo R., Kaur M., Bajic VB., Prince S. A functional SNP in the regulatory region of the decay-accelerating factor gene associates with extraocular muscle pareses in myasthenia gravis. Genes Immun. 2010. 11:1–10.

56). Nevo Y., Ben-Zeev B., Tabib A., Straussberg R., Anikster Y., Shorer Z, et al. CD59 deficiency is associated with chronic hemolysis and childhood relapsing immune-mediated polyneuropathy. Blood. 2013. 121:129–35.

57). Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009. 230:216–31.

58). Zhang H., Zhou G., Zhi L., Yang H., Zhai Y., Dong X, et al. Association between mannose-binding lectin gene polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005. 1355–61.

59). Pradhan V., Surve P., Rajadhyaksha A., Rajendran V., Patwardhan M., Umare V, et al. Mannose binding lectin (MBL) 2 gene polymorphism & its association with clinical manifestations in systemic lupus erythematosus (SLE) patients from western India. Indian J Med Res. 2015. 141:199–204.
60). Lee YH., Witte T., Momot T., Schmidt RE., Kaufman KM., Harley JB, et al. The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus: two case-control studies and a meta-analysis. Arthritis and Rheumatism. 2005. 52:3966–74.
61). Kaur S., Gupta VK., Shah A., Thiel S., Sarma PU., Madan T. Elevated levels of mannan-binding lectin [corrected] (MBL) and eosinophilia in patients of bronchial asthma with allergic rhinitis and allergic bronchopulmonary aspergillosis associate with a novel intronic polymorphism in MBL. Clin Exp Immunol. 2006. 143:414–9.
62). Zhao L., Zhang Z., Lin J., Cao L., He B., Han S, et al. Complement receptor 1 genetic variants contribute to the susceptibility to gastric cancer in Chinese population. J Cancer. 2015. 6:525–30.

63). Lambert JC., Heath S., Even G., Campion D., Sleegers K., Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009. 41:1094–9.

64). Rezzonico R., Imbert V., Chicheportiche R., Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood. 2001. 97:2932–40.
65). Park SR., Park KS., Park YJ., Band D., Lee ES. CD11a, CD11c, and CD18 gene polymorphisms and susceptibility to Behcet's disease in Koreans. Tissue Antigens. 2014. 84:398–404.
66). Degn SE., Hansen AG., Steffensen R., Jacobsen C., Jensenius JC., Thiel S. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J Immunol. 2009. 183:7371–8.

67). Endo Y., Matsushita M., Fujita T. The role of ficolins in the lectin pathway of innate immunity. Int J Biochem Cell Biol. 2011. 43:705–12.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download