Abstract
Post-transplant lymphoproliferative disorder (PTLD) is a serious complication resulting in mortality and renal graft failure. PTLD is a heterogeneous disorder, which causes different clinical forms of disease from non-specific viral syndrome to malignant lymphoma and has various etiologies, clinical features, and treatment strategies. Here, we report on a patient who had a PTLD in the hilum of a transplanted kidney at 5 months after renal transplantation. The PTLD resulted in hydronephrosis of the transplanted kidney and graft dysfunction by local urinary tract obstruction. Despite treatment including immunosuppression reduction and rituximab administration, we removed the transplanted kidney from the recipient because the PTLD did not respond to the therapy.
Go to : 
REFERENCES
1). Taal MW., Brenner BM., Rector FC. Brenner and Rector's the kidney. 9th ed.Philadelphia, PA: Saunders;2012. 2546.
2). Green M., Michaels MG. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am J Transplant. 2013. 13(Suppl 3):41–54.

3). Loren AW., Porter DL., Stadtmauer EA., Tsai DE. Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transplant. 2003. 31:145–55.

4). Mucha K., Foroncewicz B., Ziarkiewicz-Wroblewska B., Krawczyk M., Lerut J., Paczek L. Post-transplant lymphoproliferative disorder in view of the new WHO classification: a more rational approach to a protean disease? Nephrol Dial Transplant. 2010. 25:2089–98.

5). Cohen JI., Bollard CM., Khanna R., Pittaluga S. Current understanding of the role of Epstein-Barr virus in lymphoma-genesis and therapeutic approaches to EBV-associated lymphomas. Leuk Lymphoma. 2008. 49(Suppl 1):27–34.

6). Nourse JP., Jones K., Gandhi MK. Epstein-Barr Virus-related post-transplant lymphoproliferative disorders: pathogenetic insights for targeted therapy. Am J Transplant. 2011. 11:888–95.

7). Allen U., Hebert D., Moore D., Dror Y., Wasfy S., Canadian PSG. Epstein-Barr virus-related post-transplant lymphoproliferative disease in solid organ transplant recipients, 1988-97: a Canadian multi-centre experience. Pediatr Transplant. 2001. 5:198–203.

8). Yu JY., Park MY., Jeong YO., Lee HK., Park JC., Lee SJ, et al. Case report: a case of post-transplant lymphoproliferative disorder manifested as native kidney mass. Korean J Nephrol. 2009. 28:697–703. (유지연, 박미연, 정연오, 이혜경, 박지찬, 이상주, 등. 신이식 환자의 기저 신장에 발병한 posttransplant Lympoproliferative disorder (PTLD) 1예. 대한신장 학회지 2009;28: 697-703.).
9). Yoon SH., Kim SJ., Lee HW., Hwang DY., Kim JS., Cheong JW, et al. A case report of posttransplant lymphoproliferative disorders successfully treated with R-CHOP in Korea. Korean J Hematol. 2008. 43:106–12. (윤설희, 김수정, 이혜원, 황도유, 김진석, 정준원, 등. R-CHOP 요법으로 치료한 장기이식 후 림프증식 질환 2 예. 대한혈액학회지 2008;43: 106-12.).

10). Younes BS., McDiarmid SV., Martin MG., Vargas JH., Goss JA., Busuttil RW, et al. The effect of immunosuppression on posttransplant lymphoproliferative disease in pediatric liver transplant patients. Transplantation. 2000. 70:94–9.
11). Dayton JD., Richmond ME., Weintraub RG., Shipp AT., Orjuela M., Addonizio LJ. Role of immunosuppression regimen in post-transplant lymphoproliferative disorder in pediatric heart transplant patients. J Heart Lung Transplant. 2011. 30:420–5.

12). Hertig A., Zuckermann A. Rabbit antithymocyte globulin induction and risk of post-transplant lymphoproliferative disease in adult and pediatric solid organ transplantation: an update. Transpl Immunol. 2015. 32:179–87.

13). Kirk AD., Cherikh WS., Ring M., Burke G., Kaufman D., Knechtle SJ, et al. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant. 2007. 7:2619–25.

14). Robson R., Cecka JM., Opelz G., Budde M., Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005. 5:2954–60.

15). Nee R., Hurst FP., Dharnidharka VR., Jindal RM., Agodoa LY., Abbott KC. Racial variation in the development of posttransplant lymphoproliferative disorders after renal transplantation. Transplantation. 2011. 92:190–5.

16). Nepomuceno RR., Balatoni CE., Natkunam Y., Snow AL., Krams SM., Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003. 63:4472–80.
Go to : 
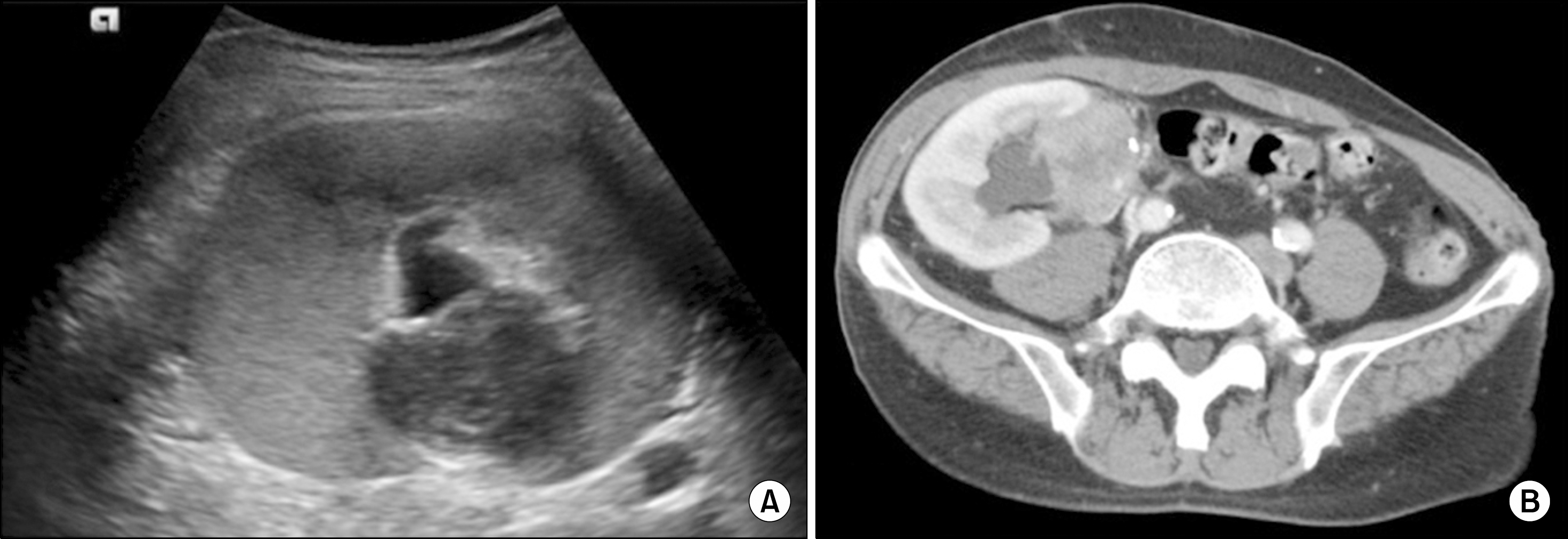 | Fig. 1.Transplanted kidney mass seen on ultrasound sonography (A) and computed tomography (B). The mass in the hilum of transplanted kidney causes hydronephrosis by local urinary tract obstruction. |
 | Fig. 2.Microscopic findings of renal mass tissue obtained by fine-needle aspiration biopsy. The tissue shows proliferation of lymphoid cells including B, T, and plasma cells (A, HE stain, ×200; B, CD20, ×200; C, CD3, ×200). Epstein Barr virus in situ hybridization shows positive staining (D, ×100). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download