Abstract
Thrombotic microangiopathy (TMA) is a serious complication of solid organ transplantation. Drug-induced TMA is typically caused by immunosuppressants, particularly calcineurin inhibitors. Withdrawing the causative drug can be one of the treatments for TMA. However, the more immunosuppressants are reduced, the more risk of rejection increases. Even if TMA is successfully resolved, the outcomes of patient and graft survival would be worse than expected. Therefore, it is necessary to maintain efficient and safe immunosuppression therapy. We report on a case of de novo TMA after kidney transplantation triggered by tacrolimus and reactivated by sirolimus. Belatacept, a novel CTLA4 Ig fusion protein, was administered for maintenance immunosuppressant with mycophenolate mofetil and prednisolon. The patient had excellent early graft outcome, and there have been no adverse events so far.
Go to : 
REFERENCES
1). Zarifian A., Meleg-Smith S., O'Donovan R., Tesi RJ., Batuman V. Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int. 1999. 55:2457–66.

2). Powles RL., Clink HM., Spence D., Morgenstern G., Watson JG., Selby PJ, et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980. 1:327–9.

3). Reynolds JC., Agodoa LY., Yuan CM., Abbott KC. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003. 42:1058–68.
4). Fortin MC., Raymond MA., Madore F., Fugere JA., Paquet M., St-Louis G, et al. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant. 2004. 4:946–52.

5). Langer RM., Van Buren CT., Katz SM., Kahan BD. De novo hemolytic uremic syndrome after kidney transplantation in patients treated with cyclosporine-sirolimus combination. Transplantation. 2002. 73:756–60.
6). Shin YT., Jung IM., Lee KW., Bae JS. Mycophenolate mofetil and prednisolone as maintenance therapy in hemolytic uremic syndrome after kidney transplantation. J Korean Soc Transplant. 1999. 13:305–10. (신영태, 정인목, 이강욱, 배진선. 신이식 후 발생한 용혈성 요독 증후군의 치료 후 유지요법으로서 Mycophenolate Mofetil과 Prednisolone의 병용요법. 대한이식 학회지 1999;13: 305-10.).
7). Taylor CM., Neild G. Acute renal failure associated with microangiopathy (haemolytic-uraemic syndrome and thrombotic thrombocytopenic purpura). Davison A, Cameron JS, Grunfeld JP, Ponticelli C, Ritz E, Winearls C, editors. ., eds.Oxford textbook of clinical nephrology. 3rd ed. Oxford: Oxford University Press:;2005. p. 1545–64.
8). Mor E., Lustig S., Tovar A., Bar-Nathan N., Shharabani E., Shapira Z, et al. Thrombotic microangiopathy early after kidney transplantation: hemolytic uremic syndrome or vascular rejection? Transplant Proc. 2000. 32:686–7.

9). Karthikeyan V., Parasuraman R., Shah V., Vera E., Venkat KK. Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant. 2003. 3:1289–94.

10). Vincenti F., Larsen C., Durrbach A., Wekerle T., Nashan B., Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005. 353:770–81.

11). Gatti S., Arru M., Reggiani P., Como G., Rossi F., Fassati LR, et al. Successful treatment of hemolytic uremic syndrome after liver-kidney transplantation. J Nephrol. 2003. 16:586–90.
12). Oyen O., Strom EH., Midtvedt K., Bentdal O., Hartmann A., Bergan S, et al. Calcineurin inhibitor-free immunosuppression in renal allograft recipients with thrombotic microangiopathy/hemolytic uremic syndrome. Am J Transplant. 2006. 6:412–8.

13). Ponticelli C., Banfi G. Thrombotic microangiopathy after kidney transplantation. Transpl Int. 2006. 19:789–94.

14). Franz M., Regele H., Schmaldienst S., Stummvoll HK., Horl WH., Pohanka E. Posttransplant hemolytic uremic syndrome in adult retransplanted kidney graft recipients: advantage of FK506 therapy? Transplantation. 1998. 66:1258–62.

15). Yango A., Morrissey P., Monaco A., Butera J., Gohh RY. Successful treatment of tacrolimus-associated thrombotic microangiopathy with sirolimus conversion and plasma exchange. Clin Nephrol. 2002. 58:77–8.

16). Naesens M., Kuypers DR., Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009. 4:481–508.

17). Ramzy D., Rao V., Tumiati LC., Xu N., Miriuka S., Delgado D, et al. Role of endothelin-1 and nitric oxide bioavailability in transplant-related vascular injury: comparative effects of rapamycin and cyclosporine. Circulation. 2006. 114(1 Suppl):I214–9.

18). Ponticelli C. De novo thrombotic microangiopathy. An underrated complication of renal transplantation. Clin Nephrol. 2007. 67:335–40.

20). Ruggenenti P., Noris M., Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001. 60:831–46.

21). Al-Massarani G., Vacher-Coponat H., Paul P., Widemann A., Arnaud L., Loundou A, et al. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. Am J Transplant. 2008. 8:2360–7.

22). Eremina V., Jefferson JA., Kowalewska J., Hochster H., Haas M., Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008. 358:1129–36.

23). Seibert FS., Steltzer J., Melilli E., Grannas G., Pagonas N., Bauer F, et al. Differential impact of belatacept and cyclosporine A on central aortic blood pressure and arterial stiffness after renal transplantation. Clin Transplant. 2014. 28:1004–9.

24). Yamada A., Salama AD., Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol. 2002. 13:559–75.

25). Wojciechowski D., Vincenti F. Challenges and opportunities in targeting the costimulation pathway in solid organ transplantation. Semin Immunol. 2011. 23:157–64.

26). Vincenti F., Charpentier B., Vanrenterghem Y., Rostaing L., Bresnahan B., Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010. 10:535–46.

27). Vincenti F., Larsen CP., Alberu J., Bresnahan B., Garcia VD., Kothari J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012. 12:210–7.

28). Ashman N., Chapagain A., Dobbie H., Raftery MJ., Sheaff MT., Yaqoob MM. Belatacept as maintenance immunosuppression for postrenal transplant de novo drug-induced thrombotic microangiopathy. Am J Transplant. 2009. 9:424–7.
Go to : 
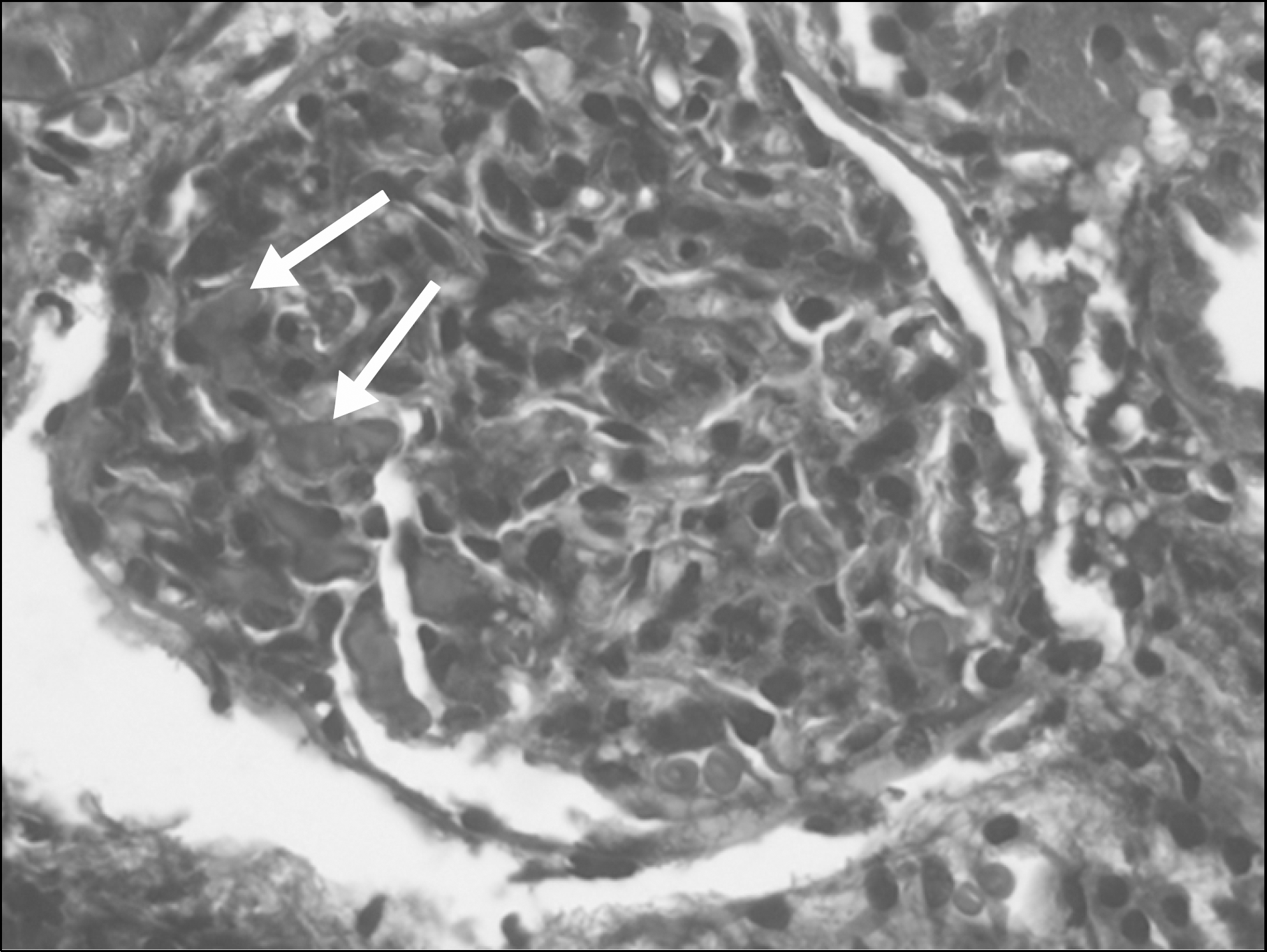
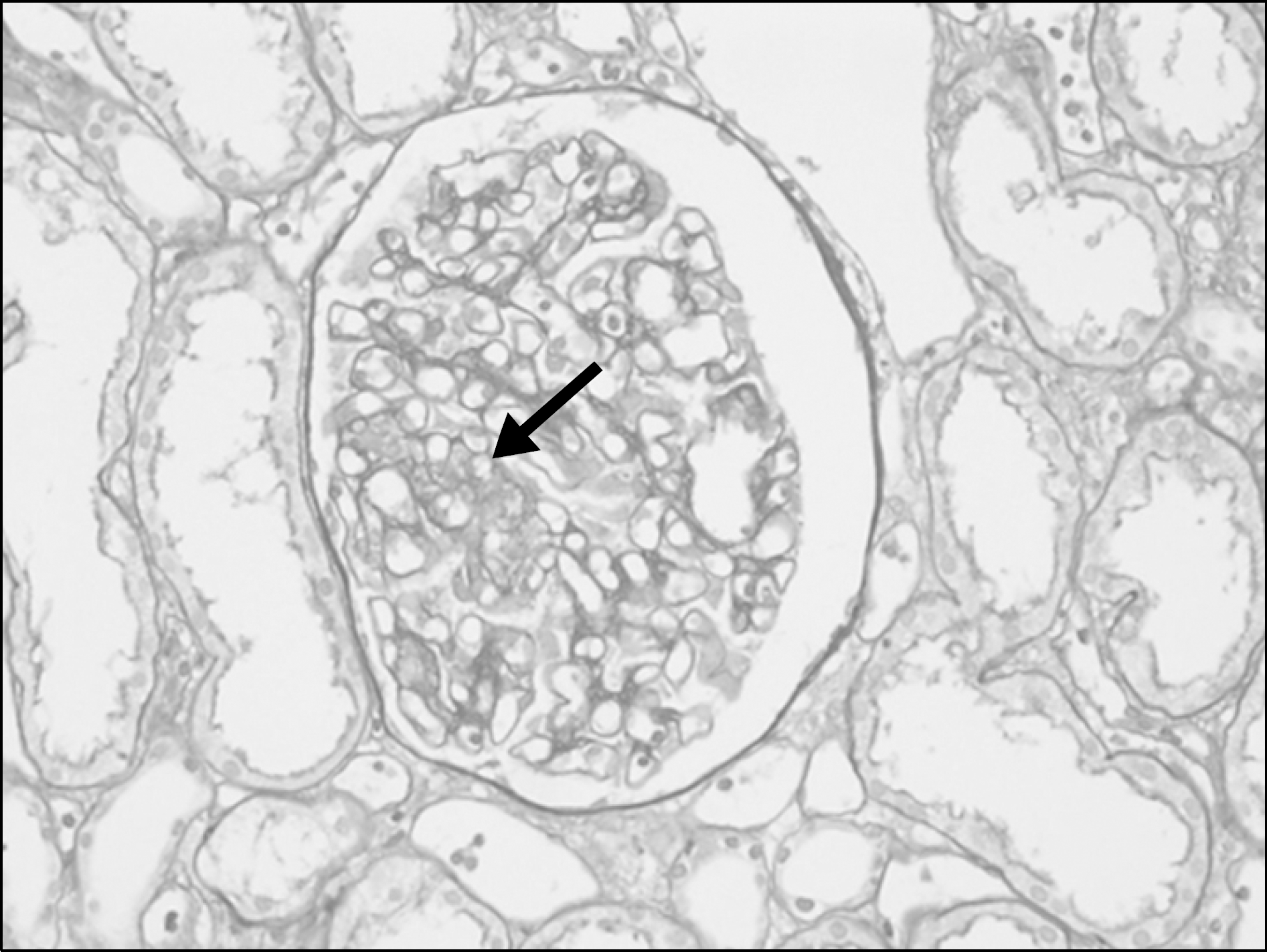
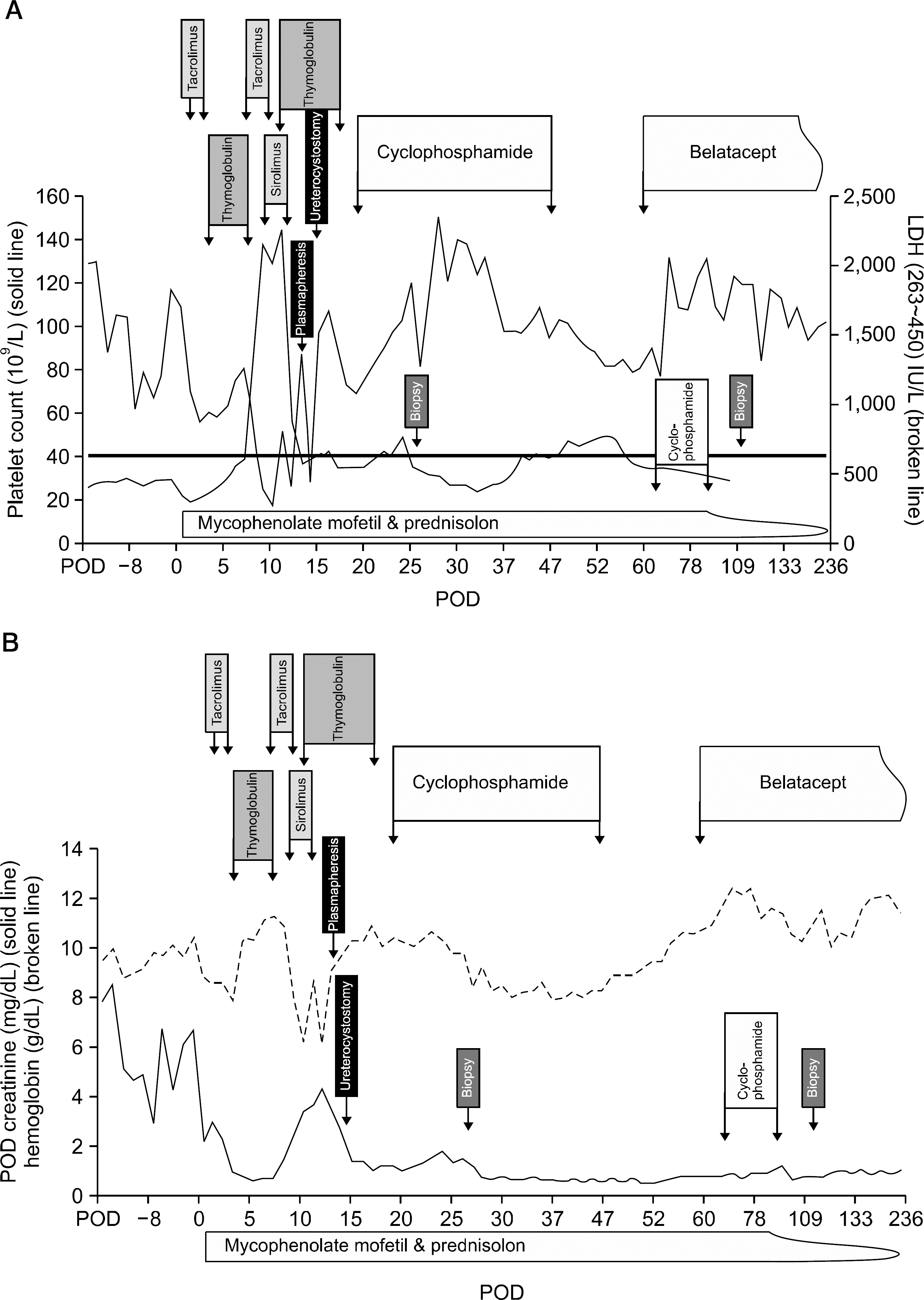 | Fig. 1.Changes in treatment progresses. (A) Platelet count and lactate dehydrogenase (LDH). (B) Creatinine and hemoglobin. (A, B) Period with the treatment: tacrolimus (1st), postoperative day (POD) 1∼2; thymoglobulin (1st), POD 3∼7; tacrolimus (2nd), POD 7∼9; sirolimus, POD 9∼ 10; thymoglobulin (2nd), POD 10∼ 17; cyclophosphamide, POD 19∼47; belatacept, POD 60≥; mycophenolate mofetil, POD 1∼; prednisolon, POD 1∼; plasmapheresis, POD 13; ureterocystostomy, POD 14; biopsy (1st), POD 26; biopsy (2nd), POD 111. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download