Abstract
Kidney transplantation (KTP) lowers the mortality and morbidity of patients with end-stage renal disease. Post-transplantation infection and antibody mediated rejection (AMR) are the most common complications. Hepatitis B surface antigen (HBsAg) positive carrier donors and high anti A/B antibody titer ABO incompatible KTP could lead to recipient hepatitis B virus (HBV) infection and AMR. Here, we report a case of successful KTP in a 41-year-old male with a high titer of ABO incompatible and HBsAg positive donor. He underwent seven rounds of plasmapheresis, low dose intravenous immunoglobulin and rituximab treatment to inhibit antibody production and remove antibodies from the serum, after which he was administered anti-viral agent for HBV prophylaxis. The recipient maintained successful allograft function for 6 months after transplantation; therefore, we report that desensitization and anti-viral treatment achieved successful outcome in a 1:512 anti A/B antibody titer ABO incompatible and hepatitis B carrier donor KTP.
Go to : 
REFERENCES
1). Kim MH., Kim MS., Kwon OJ., Kang CM. Comparison of quality of life between kidney transplant patients and dialysis patients. J Korean Soc Transplant. 2009. 23:65–70. (김명 희, 김민수, 권오정, 강종명. 신장이식환자와 투석환자의 삶의 질 비교. 대한이식학회지 2009;23: 65-70.).

2). Ogutmen B., Yildirim A., Sever MS., Bozfakioglu S., Ataman R., Erek E, et al. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc. 2006. 38:419–21.

3). Tonelli M., Wiebe N., Knoll G., Bello A., Browne S., Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011. 11:2093–109.

4). Ojo AO., Hanson JA., Meier-Kriesche H., Okechukwu CN., Wolfe RA., Leichtman AB, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001. 12:589–97.

5). Almond PS., Matas A., Gillingham K., Dunn DL., Payne WD., Gores P, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993. 55:752–6.
6). Peterson PK., Balfour HH Jr., Marker SC., Fryd DS., Howard RJ., Simmons RL. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore). 1980. 59:283–300.
7). Kasiske BL. Risk factors for accelerated atherosclerosis in renal transplant recipients. Am J Med. 1988. 84:985–92.

8). Kasiske BL., Snyder JJ., Gilbertson DT., Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004. 4:905–13.

9). Cosio FG., Kudva Y., van der Velde M., Larson TS., Textor SC., Griffin MD, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005. 67:2415–21.

10). Tanabe K., Takahashi K., Sonda K., Tokumoto T., Ishikawa N., Kawai T, et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation. 1998. 65:224–8.

12). Patel R., Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969. 280:735–9.

13). Suehiro T., Shimada M., Kishikawa K., Shimura T., Soejima Y., Yoshizumi T, et al. Influence of HLA compatibility and lymphocyte cross-matching on acute cellular rejection following living donor adult liver transplantation. Liver Int. 2005. 25:1182–8.

14). Natov SN., Pereira BJ. Transmission of viral hepatitis by kidney transplantation: donor evaluation and transplant policies (part 1: hepatitis B virus). Transpl Infect Dis. 2002. 4:117–23.
15). Warren DS., Zachary AA., Sonnenday CJ., King KE., Cooper M., Ratner LE, et al. Successful renal transplantation across simultaneous ABO incompatible and positive crossmatch barriers. Am J Transplant. 2004. 4:561–8.

16). Murray JE., Reid DE., Harrison JH., Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med. 1963. 269:341–3.

17). McKay DB., Josephson MA., Armenti VT., August P., Coscia LA., Davis CL, et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant. 2005. 5:1592–9.

18). Kramer L., Madl C., Stockenhuber F., Yeganehfar W., Eisen-huber E., Derfler K, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996. 49:833–8.

19). van den Ham EC., Kooman JP., Schols AM., Nieman FH., Does JD., Franssen FM, et al. Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. Am J Transplant. 2005. 5:1957–65.

20). Lutwick LI., Sywassink JM., Corry RJ., Shorey JW. The trans-mission of hepatitis B by renal transplantation. Clin Nephrol. 1983. 19:317–9.
21). Wolf JL., Perkins HA., Schreeder MT., Vincenti F. The transplanted kidney as a source of hepatitis B infection. Ann Intern Med. 1979. 91:412–3.

22). Scornik JC., Clapp W., Patton PR., Van der Werf WJ., Hemming AW., Reed AI, et al. Outcome of kidney transplants in patients known to be flow cytometry crossmatch positive. Transplantation. 2001. 71:1098–102.
23). Piazza A., Poggi E., Borrelli L., Servetti S., Monaco PI., Buonomo O, et al. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cytometric techniques. Transplantation. 2001. 71:1106–12.
24). Schlaf G., Mauz-Korholz C., Ott U., Leike S., Altermann W. General insufficiency of the classical CDC-based crossmatch to detect donor-specific anti-HLA antibodies leading to invalid results under recipients' medical treatment or underlying diseases. Histol Histopathol. 2012. 27:31–8.
25). Won DI. Experimental application of whole blood flow cytometry to HLA crossmatch for renal transplantation. Korean J Lab Med. 2006. 26:45–51. (원동일. 신장 이식을 위한 HLA 교차시험에서 전혈 유세포분석법의 실험적 적용. 대한진단 검사의학회지 2006;26: 45-51.).

26). Stegall MD., Dean PG., Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004. 78:635–40.

27). Tobian AA., Shirey RS., Montgomery RA., Cai W., Haas M., Ness PM, et al. ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant. 2010. 10:1247–53.

28). Montgomery RA. ABO incompatible transplantation: to B or not to B. Am J Transplant. 2004. 4:1011–2.

29). Vo AA., Lukovsky M., Toyoda M., Wang J., Reinsmoen NL., Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008. 359:242–51.

30). Alexandre GP., Squifflet JP., De Bruyere M., Latinne D., Moriau M., Ikabu N, et al. Splenectomy as a prerequisite for successful human ABO-incompatible renal-transplantation. Transplant Proc. 1985. 17:138–43.
31). Tyden G., Kumlien G., Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003. 76:730–1.
32). Tanabe K., Ishida H., Shimizu T., Omoto K., Shirakawa H., Tokumoto T. Evaluation of two different preconditioning regimens for ABO-incompatible living kidney donor transplantation. A comparison of splenectomy vs. rituximab-treated non-splenectomy preconditioning regimens. Contrib Nephrol. 2009. 162:61–74.
33). Lee J., Lee JG., Kim S., Song SH., Kim BS., Kim HO, et al. The effect of rituximab dose on infectious complications in ABO-incompatible kidney transplantation. Nephrol Dial Transplant. 2016. 31:1013–21.

34). Chung RT., Feng S., Delmonico FL. Approach to the management of allograft recipients following the detection of hepatitis B virus in the prospective organ donor. Am J Transplant. 2001. 1:185–91.

35). Kang MG., Lee SJ., Oh JS., Lim YA. Comparison of ABO iso-agglutinin titers by different tube hemagglutination techniques. Korean J Blood Transfus. 2009. 20:227–34. (강민구, 이승재, 오진숙, 임영애. 시험관법을 이용한 검사법에 따른 ABO 동종 응집소 역가 비교. 대한수혈학회지 2009;20: 227-34.).
Go to : 
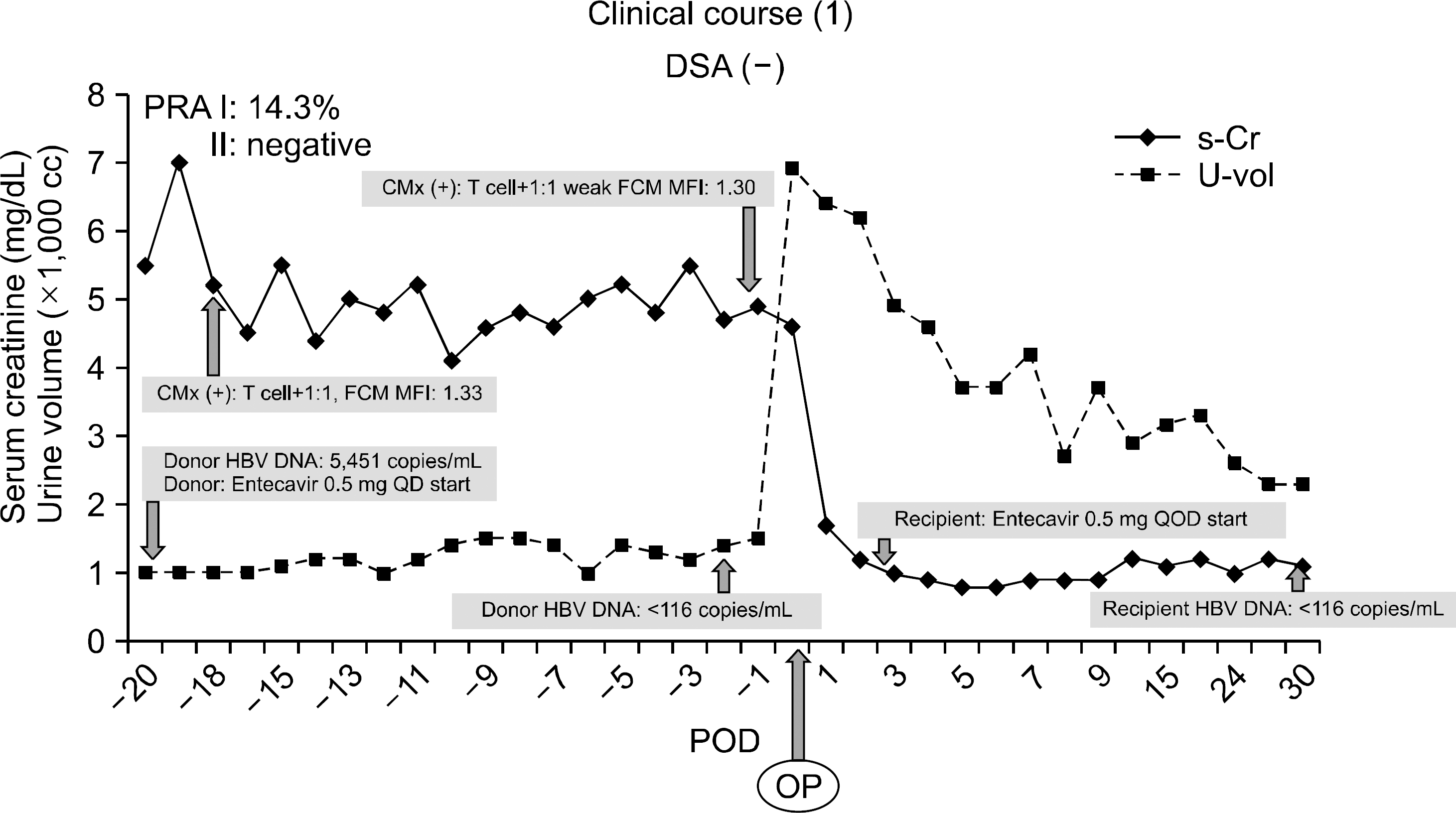 | Fig. 1.Changes of creatinine and urine volume before and after transplantation. Abbreviations: DSA, donor specific antibody; PRA, panel reactive antibody; CMx, cross matching; FCM, flow cytometry; MFI, mean fluorescence intensity; HBV, hepatitis B virus; QD, once a day; QOD, every other day; POD, postoperation day; OP, operation. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download