Abstract
Hepatitis C virus (HCV)-related liver disease is the most common indication for liver transplantation (LT) in Western countries, whereas HCV LT is rare in Korea. We conducted a survey of HCV RNA-positive patients who underwent LT and investigated the prognostic factors for patient survival and the effects of immunosuppression. To accomplish this, we retrospectively reviewed the multicenter records of 192 HCV RNA-positive patients who underwent LT. The 1-, 3-, and 5-year overall survival rates were 78.8%, 75.3%, and 73.1%, respectively. Excluding cases of hospital mortality (n=23), 169 patients were evaluated. Most patients were genotype 1 (n=111, 65.7%) or genotype 2 (n=42, 24.9%). The proportion of living donors for LT (n=135, 79.9%) was higher than that of deceased donors (DDLT; n=34, 20.1%). The median donor and recipient ages were 32 and 56 years, respectively. Twenty-eight patients (16.6%) died during the observation period, while 75 underwent universal prophylaxis and 15 received preemptive therapy. HCV recurrence was detected in 97 patients. Recipients who were older than 60, received DDLT, used cyclosporine, or suffered acute rejection had lower rates of survival. Acute rejection was closely associated with a lack of induction therapy, cyclosporine use, and universal prophylaxis after transplantation. The careful avoidance of acute rejection in the post-transplant period through adequate use of tacrolimus is a preferable strategy that increases patient survival following liver transplantation.
REFERENCES
1). Mohd Hanafiah K., Groeger J., Flaxman AD., Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013. 57:1333–42.

2). European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011. 55:245–64.
3). Shin HR., Hwang SY., Nam CM. The prevalence of hepatitis C virus infection in Korea: pooled analysis. J Korean Med Sci. 2005. 20:985–8.

4). Kim SJ. Viral hepatitis surveillance system and statue of C hepatitis sentinel surveillance in Korea. Public Health Weekly Report, KCDC. 2012. 214–9.
5). Lee HW., Lee KW., Kim BW., Song GW., Han YS., Kwon CH, et al. Liver transplantation for hepatitis C virus-related liver disease in Korea. J Korean Soc Transplant. 2012. 26:269–76.

6). Balagopal A., Thomas DL., Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010. 139:1865–76.

7). Gane EJ., Naoumov NV., Qian KP., Mondelli MU., Maertens G., Portmann BC, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996. 110:167–77.

8). Howell J., Angus P., Gow P. Hepatitis C recurrence: the Achilles heel of liver transplantation. Transpl Infect Dis. 2014. 16:1–16.

9). Gane EJ., Portmann BC., Naoumov NV., Smith HM., Underhill JA., Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996. 334:815–20.

10). Berenguer M., Prieto M., San Juan F., Rayón JM., Martinez F., Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002. 36:202–10.

11). Samonakis DN., Germani G., Burroughs AK. Immunosuppression and HCV recurrence after liver transplantation. J Hepatol. 2012. 56:973–83.

12). Everson GT., Trotter J., Forman L., Kugelmas M., Halprin A., Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005. 42:255–62.

13). Singal AK., Guturu P., Hmoud B., Kuo YF., Salameh H., Wiesner RH. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013. 95:755–60.

14). Adam R., Karam V., Delvart V., O'Grady J., Mirza D., Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012. 57:675–88.

15). Akamatsu N., Sugawara Y., Kokudo N., Eguchi S., Fujiwara T., Ohdan H, et al. Outcomes of living donor liver transplantation for hepatitis C virus-positive recipients in Japan: results of a nationwide survey. Transpl Int. 2014. 27:767–74.

16). Charlton M., Seaberg E., Wiesner R., Everhart J., Zetterman R., Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998. 28:823–30.

17). Shackel NA., Jamias J., Rahman W., Prakoso E., Strasser SI., Koorey DJ, et al. Early high peak hepatitis C viral load levels independently predict hepatitis C-related liver failure post-liver transplantation. Liver Transpl. 2009. 15:709–18.

18). Roche B., Samuel D. Hepatitis C virus treatment pre- and post-liver transplantation. Liver Int. 2012. 32(Suppl 1):120–8.

19). Chalasani N., Manzarbeitia C., Ferenci P., Vogel W., Fontana RJ., Voigt M, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005. 41:289–98.

20). Bzowej N., Nelson DR., Terrault NA., Everson GT., Teng LL., Prabhakar A, et al. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011. 17:528–38.

21). Guillouche P., Féray C. Systematic review: antiviral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011. 33:163–74.

22). Berenguer M., Rayón JM., Prieto M., Aguilera V., Nicolás D., Ortiz V, et al. Are posttransplantation protocol liver biopsies useful in the long term? Liver Transpl. 2001. 7:790–6.

23). Berenguer M., Palau A., Aguilera V., Rayón JM., Juan FS., Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008. 8:679–87.

24). Fukuhara T., Taketomi A., Motomura T., Okano S., Ninomiya A., Abe T, et al. Variants in IL28B in liver recipients and donors correlate with response to peginterferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010. 139:1577–85. 1585 e1-3.

25). Watashi K., Hijikata M., Hosaka M., Yamaji M., Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003. 38:1282–8.

26). Carrión JA., Navasa M., García-Retortillo M., García-Pagan JC., Crespo G., Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007. 132:1746–56.

27). Rabie R., Mumtaz K., Renner EL. Efficacy of antiviral therapy for hepatitis C after liver transplantation with cyclosporine and tacrolimus: a systematic review and meta-analysis. Liver Transpl. 2013. 19:36–48.

28). ReViS-TC Study Group. Cyclosporine a-based immunosuppression reduces relapse rate after antiviral therapy in transplanted patients with hepatitis C virus infection: a large multicenter cohort study. Transplantation. 2011. 92:334–40.
29). Martin P., Busuttil RW., Goldstein RM., Crippin JS., Klintmalm GB., Fitzsimmons WE, et al. Impact of tacrolimus versus cyclosporine in hepatitis C virus-infected liver transplant recipients on recurrent hepatitis: a prospective, randomized trial. Liver Transpl. 2004. 10:1258–62.

30). Berenguer M., Royuela A., Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007. 13:21–9.

Fig. 1.
Patient survival rates. The 1-, 3-, and 5-year patient survival rates are 78.8%, 75.3%, and 73.1%, respectively.
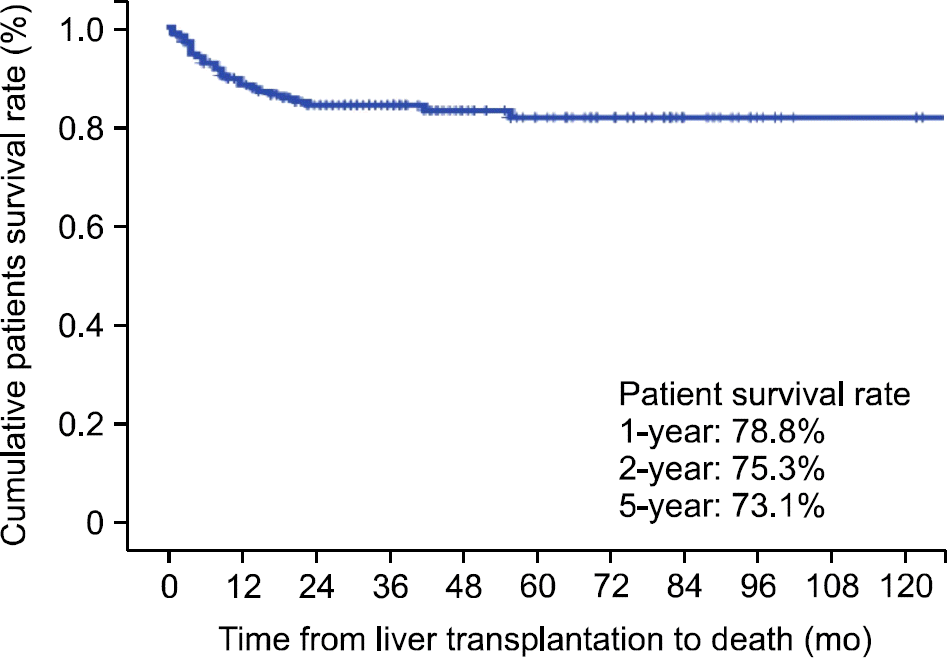
Fig. 2.
Patient survival according to recipient age, donor type, calcineurin inhibitor, and biopsy-proven acute rejection. Abbreviations: LDLT, living donor liver transplantation; DDLT, deceased donor liver transplantation; BPAR, biopsy-proven acute rejection.
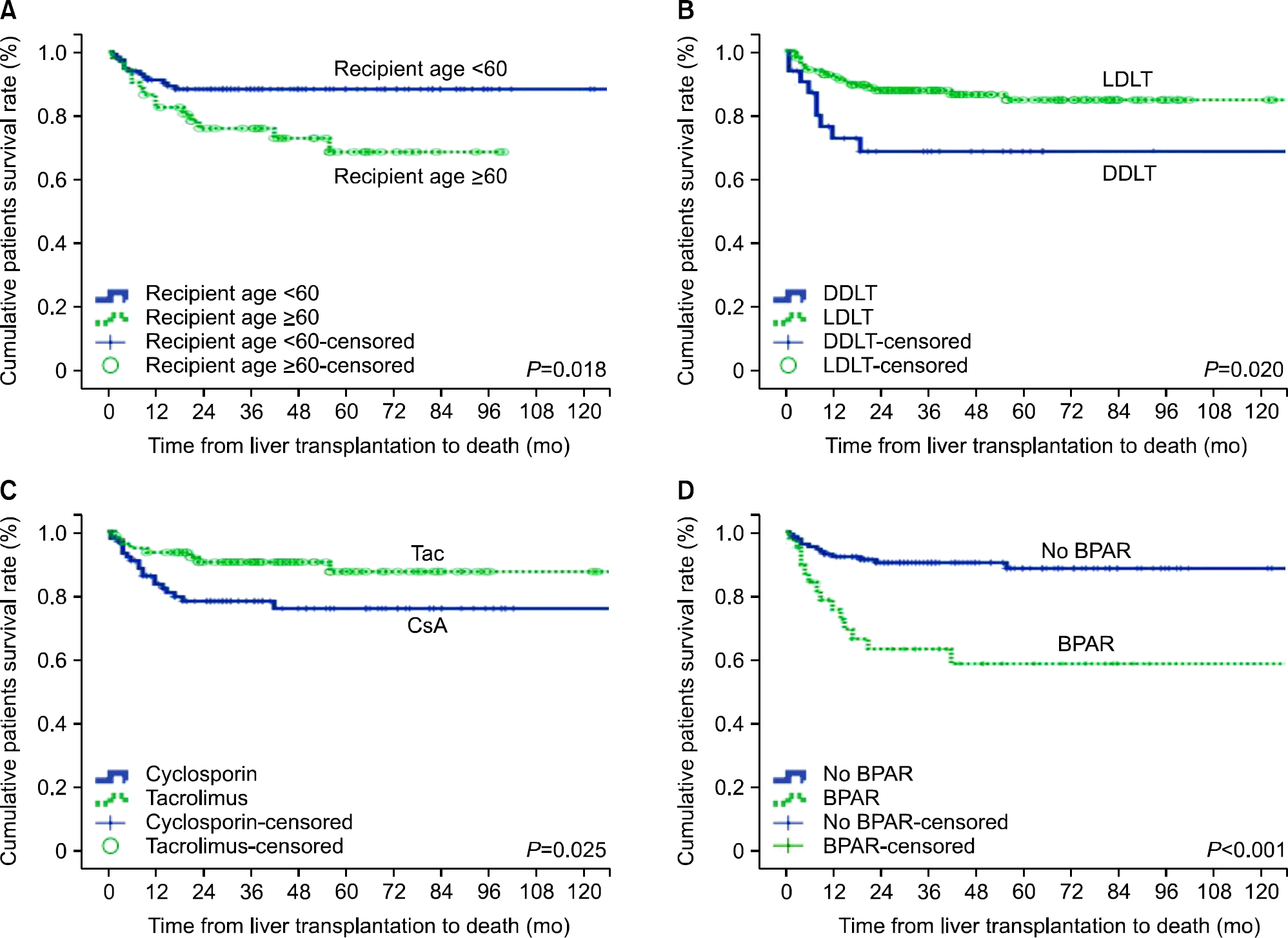
Fig. 3.
Patient survival according to SVR. Patient survival in patients with SVR was higher than in patients without SVR, but there was no statistically significant difference in patient survival between the two groups. Abbreviations: SVR, sustained viral response.
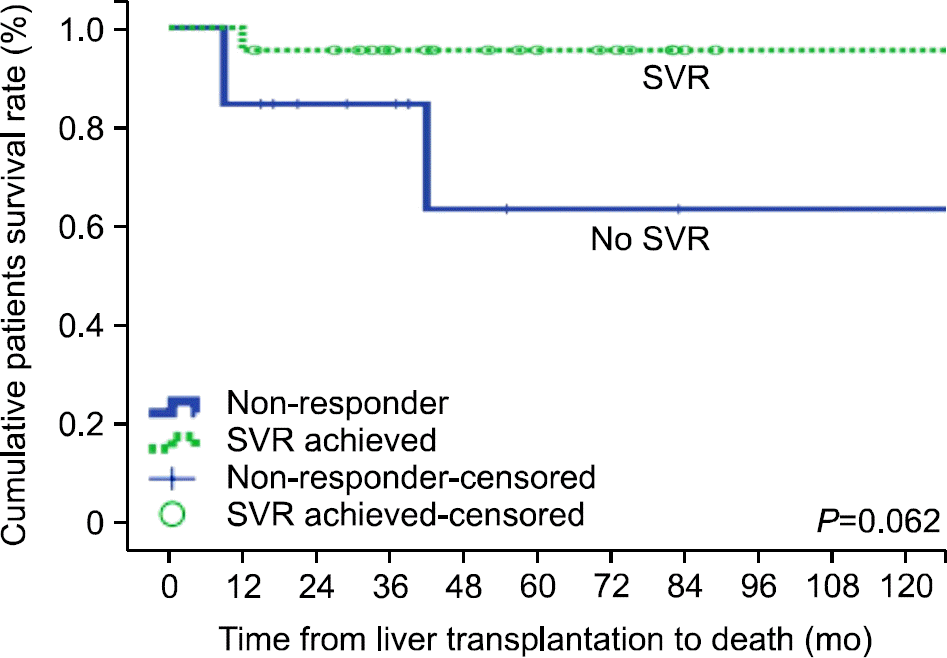
Table 1.
The causes of graft failure and mortality
Table 2.
Baseline characteristics
Table 3.
Risk factors for patient survival
Table 4.
Antiviral treatment in pre- and post-transplant
Table 5.
Comparison of patients with and without biopsy-proven acute rejection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download