Abstract
Background
The aim of this study is to evaluate the clinical outcomes between anti-thymocyte globulin (ATG) and basiliximab induction in deceased donor kidney transplantation (DDKT).
Methods
Between May 2006 and February 2015, 40 patients underwent DDKT at our institution. Three cases (7.5%) of them were lost during the following-up schedule. In this study, ATG induction criteria were donor age >50 years old or donor creatinine level >1.3 mg/dL except hepatitis B virus positive and hepatitis C virus positive recipients. Recipients were divided into two groups: the ATG group (n=20) and the basiliximab group (n=17).
Results
The 1-year patient survival in the ATG group was 89.4% compared to 93.8% in the basiliximab group (P=0.989). Graft survival for a 1 year in the ATG and the basiliximab group was 89.1% and 93.8%, respectively (P=0.967). Incidences of acute rejection episodes were more prevalent in the basiliximab group (15.0% vs. 29.4%, P=0.428). The glomerular filtration rate level by period of recipients was not different in both group (12th month, 64.60±16.17 mg/dL vs. 68.51±18.60 mg/dL, P=0.544). The overall complications during the follow-up were not significantly different in both groups (90.0% vs. 76.5%, P=0.383).
Conclusions
The results showed that there was no difference in the patient survival and graft survival between induction of ATG and basiliximab of the DDKT were not different. Therefore, use of both induction agents led to a good patient and graft survival and ATG might be a safe and preferable agent for relatively poor renal function of donor in kidney transplantation.
Go to : 
References
1). Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006; 355:1967–77.

2). Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a metaanalysis. Transplant Proc. 2010; 42:1667–70.

3). Neidlinger NA, Sollinger HW. Is there any role for antithymocyte induction in renal transplantation? Transplant Proc. 2010; 42:1402–7.

4). Sollinger H, Kaplan B, Pescovitz MD, Philosophe B, Roza A, Brayman K, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation. 2001; 72:1915–9.
5). Wang W, Yin H, Li XB, Hu XP, Yang XY, Liu H, et al. A retrospective comparison of the efficacy and safety in kidney transplant recipients with basiliximab and antithymocyte globulin. Chin Med J (Engl). 2012; 125:1135–40.
6). Yang SL, Wang D, Wu WZ, Lin WH, Xu TZ, Cai JQ, et al. Comparison of single bolus ATG and Basiliximab as induction therapy in presensitized renal allograft recipients receiving tacrolimus-based immunosuppressive regimen. Transpl Immunol. 2008; 18:281–5.

7). Nashan B. Antibody induction therapy in renal transplant patients receiving calcineurin-inhibitor immunosuppressive regimens: a comparative review. BioDrugs. 2005; 19:39–46.
8). Duzova A, Buyan N, Bakkaloglu M, Dalgic A, Soylemezoglu O, Besbas N, et al. Triple immunosuppression with or without basiliximab in pediatric renal transplantation: acute rejection rates at one year. Transplant Proc. 2003; 35:2878–80.

9). Kim JM, Jang HR, Kwon CH, Huh WS, Kim GS, Kim SJ, et al. Rabbit antithymocyte globulin compared with basiliximab in kidney transplantation: a singlecenter study. Transplant Proc. 2012; 44:167–70.

10). Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. ATG versus basiliximab induction therapy in renal allograft recipients receiving a dual immunosuppressive regimen: one-year results. Transplant Proc. 2003; 35:2100–1.

11). Foster CE 3rd, Weng RR, Piper M, Laugenou K, Ichii H, Lakey J, et al. Induction therapy by antithymocyte globulin (rabbit) versus basiliximab in deceased donor renal transplants and the effect on delayed graft function and outcomes. Transplant Proc. 2012; 44:164–6.

12). Ulrich F, Niedzwiecki S, Pascher A, Kohler S, Weiss S, Fikatas P, et al. Long-term outcome of ATG vs. basiliximab induction. Eur J Clin Invest. 2011; 41:971–8.

13). Lebranchu Y, Bridoux F, Buchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant. 2002; 2:48–56.

14). Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation. 2004; 78:584–90.

15). Pilch NA, Taber DJ, Moussa O, Thomas B, Denmark S, Meadows HB, et al. Prospective randomized controlled trial of rabbit antithymocyte globulin compared with IL-2 receptor antagonist induction therapy in kidney transplantation. Ann Surg. 2014; 259:888–93.

16). Laftavi MR, Alnimri M, Weber-Shrikant E, Kohli R, Said M, Patel S, et al. Low-dose rabbit antithymocyte globulin versus basiliximab induction therapy in low-risk renal transplant recipients: 8-year follow-up. Transplant Proc. 2011; 43:458–61. 2011;43: 458–61.17) Son YK, Oh JS, Oh HJ, Shin YH, Kim JK, Jeong HJ. Leflunomide treatment in BK virus associated nephropathy.

18). Barri YM, Ahmad I, Ketel BL, Barone GW, Walker PD, Bonsib SM, et al. Polyoma viral infection in renal transplantation: the role of immunosuppressive therapy. Clin Transplant. 2001; 15:240–6.

20). Imperiale MJ, Major EO. Polyomaviruses. Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th ed.Vol. 2. Philadelphia: Lippincott Williams & Wilkins;2007. p. 2263.
21). Shah KV. Polyomaviruses. Fields BN, Knipe DM, Howley PM, editors. Fields virology. 3rd ed.Vol. 2. Philadelphia: Lippincott-Raven Publishers;1996. : 2027.
22). Boldorini R, Veggiani C, Barco D, Monga G. Kidney and urinary tract polyomavirus infection and distribution: molecular biology investigation of 10 consecutive autopsies. Arch Pathol Lab Med. 2005; 129:69–73.

23). Jin L, Gibson PE, Booth JC, Clewley JP. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol. 1993; 41:11–7.

24). Dadhania D, Snopkowski C, Ding R, Muthukumar T, Chang C, Aull M, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008; 86:521–8.

25). Gautam A, Patel V, Pelletier L, Orozco J, Francis J, Nuhn M. Routine BK virus surveillance in renal transplantation: a single center's experience. Transplant Proc. 2010; 42:4088–90.
Go to : 
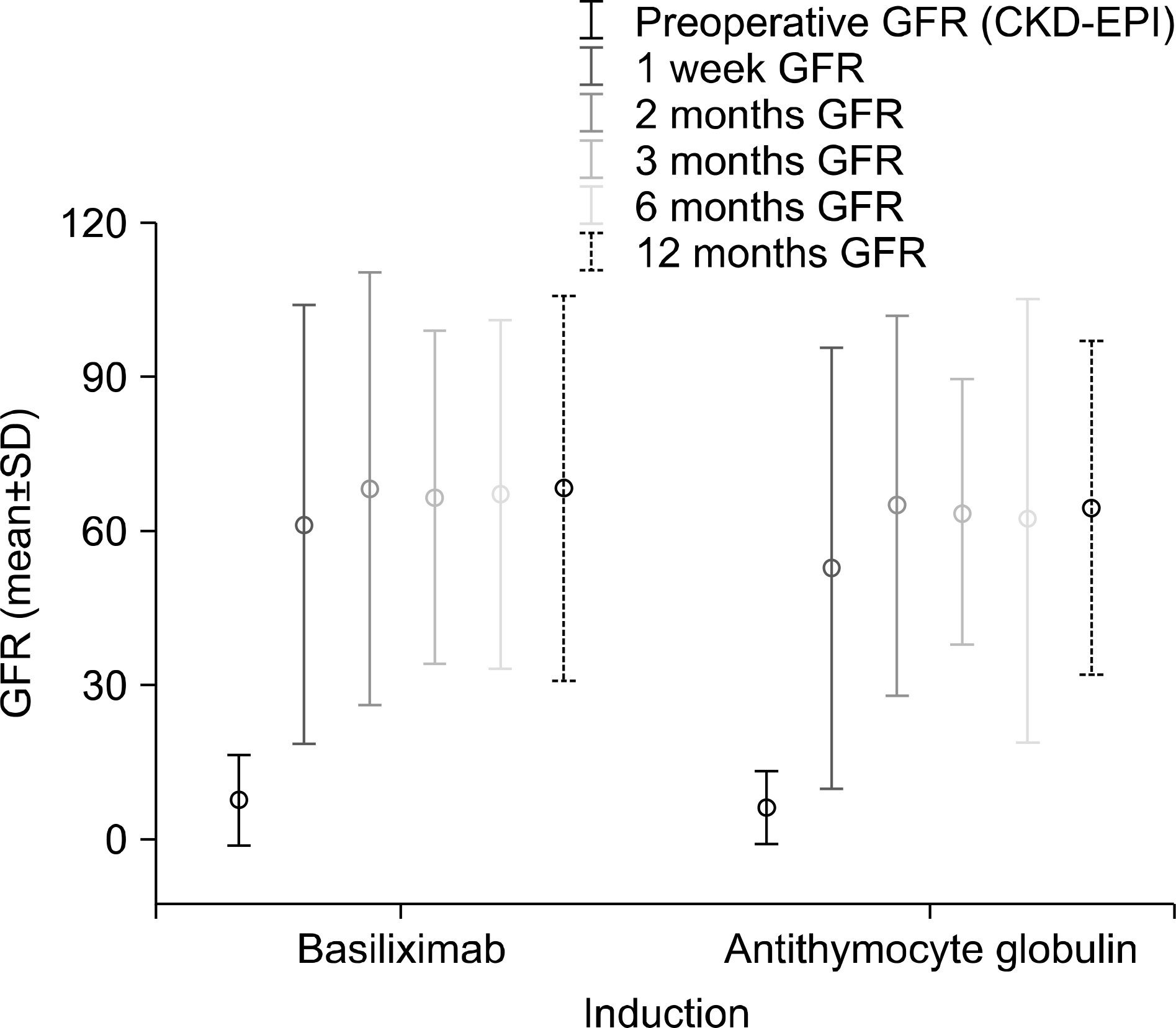 | Fig. 1.Comparison of the glomerular filtration rate (GFR) (Chronic Kidney Disease Epidemiology Collaboration, CKD-EPI) level by period after induction of antithymocyte globulin versus basiliximab. GFR level by period of recipients was not different in both group (P=0.544 at 12th month). |
Table 1.
Demographic and clinical data
Table 2.
Comparison of the postoperative complications after induction of ATG versus basiliximab
Table 3.
Comparison of the postoperative patient survival and graft survival after induction of ATG versus basiliximab
| Variable | ATG (n=20) | Basiliximab (n=17) | P-value |
|---|---|---|---|
| Delayed graft function | 3 (15.0) | 2 (11.8) | 1.000 |
| Acute T-cell mediated rejection | 3 (15.0) | 5 (29.4) | 0.428 |
| Antibody mediated rejection | 1 (5.0) | 1 (5.9) | 1.000 |
| Graft loss | 1 (5.0) a | 1 (5.9) b | 1.000 |
| Death | 2 (10.0) | 2 (11.8) | 1.000 |
| 1 Year patient survival rate (%) | 89.4 | 93.8 | 0.989 |
| 1 Year graft survival rate (%) | 89.1 | 93.8 | 0.967 |
| 1 Year graft survival rate c (%) | 94.7 | 100.0 | 0.344 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download