Abstract
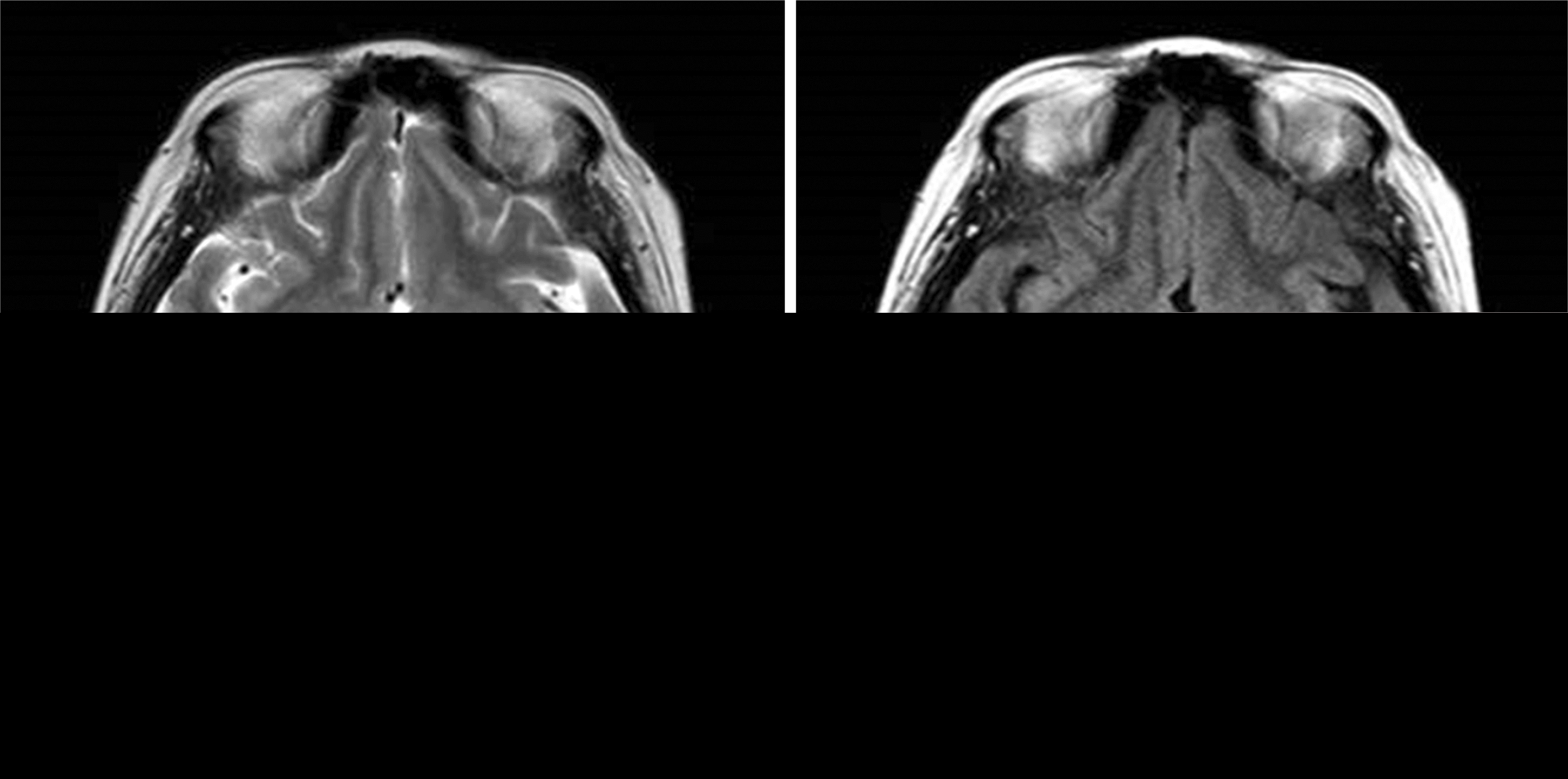
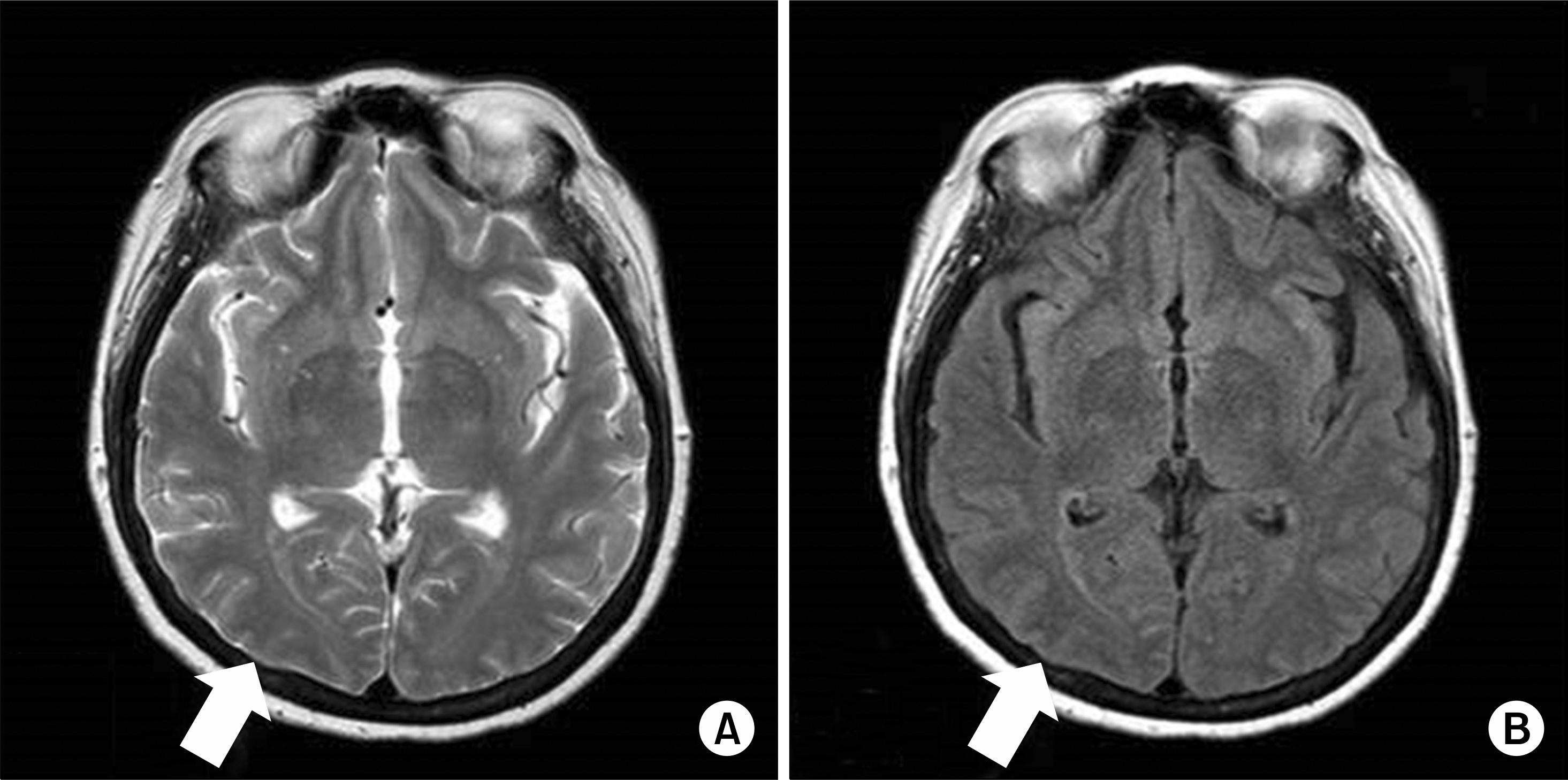
Posterior reversible encephalopathy syndrome (PRES) is neurotoxicity characterized by brain imaging findings of reversible sub-cortical vasogenic edema. Clinical manifestations include seizure, altered mental status, focal neurologic deficit, and headache. Tacrolimus, a potent immunosuppressant, is related to increased risk of PRES in transplantation recipients. We report on a case of PRES in a 48-year-old female kidney transplantation recipient who received immunosuppression with tacrolimus, mycophenolate mofetil, and prednisolone. On postoperative day 14, she complained of moderate to severe headache which did not respond to usual analgesics. Magnetic resonance imaging showed high signal intensity on T2-weighted images and fluid-attenuated inverse recovery imaging in both parieto-occipital areas. The condition was improved after changing immunosuppressant from tacrolimus to sirolimus.
Go to : 
References
1). Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500.

2). Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008; 29:1036–42.

3). Bartynski WS, Tan HP, Boardman JF, Shapiro R, Marsh JW. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol. 2008; 29:924–30.

4). Wong R, Beguelin GZ, de Lima M, Giralt SA, Hosing C, Ippoliti C, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2003; 122:128–34.

5). Wu Q, Marescaux C, Wolff V, Jeung MY, Kessler R, Lauer V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol. 2010; 64:169–77.

6). Hammerstrom AE, Howell J, Gulbis A, Rondon G, Champlin RE, Popat U. Tacrolimus-associated posterior reversible encephalopathy syndrome in hematopoietic allogeneic stem cell transplantation. Am J Hematol. 2013; 88:301–5.

7). Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010; 85:427–32.

8). Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008; 65:205–10.

9). Singh N, Bonham A, Fukui M. Immunosuppressive-associated leukoencephalopathy in organ transplant recipients. Transplantation. 2000; 69:467–72.

10). Besenski N, Rumboldt Z, Emovon O, Nicholas J, Kini S, Milutinovic J, et al. Brain MR imaging abnormalities in kidney transplant recipients. AJNR Am J Neuroradiol. 2005; 26:2282–9.
11). Rykken JB, McKinney AM. Posterior reversible encephalopathy syndrome. Semin Ultrasound CT MR. 2014; 35:118–35.

12). Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008; 29:1043–9.

13). Moskowitz A, Nolan C, Lis E, Castro-Malaspina H, Perales MA. Posterior reversible encephalopathy syndrome due to sirolimus. Bone Marrow Transplant. 2007; 39:653–4.

14). Dzudie A, Boissonnat P, Roussoulieres A, Cakmak , Mosbah K, Bejui FT, et al. Cyclosporine-related posterior reversible encephalopathy syndrome after heart transplantation: should we withdraw or reduce cyclosporine?: case reports. Transplant Proc. 2009; 41:716–20.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download