Abstract
Background
The aim of this study is to evaluate the feasibility of living donor liver transplantation (LDLT) using an right posterior sector (RPS) graft selected by liver volumetry of living donors.
Methods
From April 2008 to August 2014, 132 LDLTs were performed in our hospital. Of these, 20 recipients (15.1%) received an RPS graft. Perioperative data of LDLTs using an RPS graft were analyzed retrospectively.
Results
Mean of the Model for End-stage Liver Disease score of the 20 recipients was 12.1±6.2. The mean right liver volume was 72.4%±3.1% of total liver volume (TLV) and the mean volume of RPS was 38.2%±5.3% of TLV. Anatomical anomalies were found in the portal vein (PV) of 14 donors (70%), in the hepatic artery of one donor (5%), and bile duct of seven donors (35%). All donors were discharged with normal liver function. Two donors (10%) developed bile leakage after RPS donation. None of the recipients experienced complication associated with hepatic artery and PV anastomosis. One recipient had in-hospital mortality due to pneumonia. The remaining 19 recipients were discharged with good graft function. Four recipients (20%) developed biliary stricture and one (5%) had a liver abscess during follow-up.
Conclusions
The RPS donor had a high incidence of abnormal anatomy of PV. LDLT using an RPS graft might have high incidence of biliary complications. We think that selection of an RPS graft from a donor with an inappropriately large right lobe volume could expand the donor pool and be a feasible option in LDLT.
Go to : 
References
1). Gali B, Rosen CB, Plevak DJ. Living donor liver transplantation: selection, perioperative care, and outcome. J Intensive Care Med. 2012; 27:71–8.
2). Campos BD, Botha JF. Strategies to optimize donor safety with smaller grafts for adult-to-adult living donor liver transplantation. Curr Opin Organ Transplant. 2012; 17:230–4.

3). Barr ML, Belghiti J, Villamil FG, Pomfret EA, Sutherland DS, Gruessner RW, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006; 81:1373–85.

4). Kim BW, Xu W, Wang HJ, Park YK, Lee K, Kim MW. Volumetry-based selection of right posterior sector grafts for adult living donor liver transplantation. Liver Transpl. 2011; 17:1046–58.

5). Yoshizumi T, Ikegami T, Kimura K, Uchiyama H, Ikeda T, Shirabe K, et al. Selection of a right posterior sector graft for living donor liver transplantation. Liver Transpl. 2014; 20:1089–96.

6). Hori T, Kirino I, Uemoto S. Right posterior segment graft in living donor liver transplantation. Hepatol Res. Epub. 2015 Jan 6. DOI:. http://dx.doi.org/10.1111/hepr.12469.
7). Hwang S, Lee SG, Lee YJ, Park KM, Kim KH, Ahn CS, et al. Donor selection for procurement of right posterior segment graft in living donor liver transplantation. Liver Transpl. 2004; 10:1150–5.

8). Sugawara Y, Makuuchi M, Takayama T, Mizuta K, Kawarasaki H, Imamura H, et al. Liver transplantation using a right lateral sector graft from a living donor to her granddaughter. Hepatogastroenterology. 2001; 48:261–3.
9). Kyoden Y, Tamura S, Sugawara Y, Akamatsu N, Matsui Y, Togashi J, et al. Biliary complications in right lateral sector graft live donor liver transplantation. Transpl Int. 2008; 21:332–9.

10). Marubashi S, Dono K, Nagano H, Kobayashi S, Takeda Y, Umeshita K, et al. Biliary reconstruction in living donor liver transplantation: technical invention and risk factor analysis for anastomotic stricture. Transplantation. 2009; 88:1123–30.

11). Leelaudomlipi S, Sugawara Y, Kaneko J, Matsui Y, Ohkubo T, Makuuchi M. Volumetric analysis of liver segments in 155 living donors. Liver Transpl. 2002; 8:612–4.

12). Sugawara Y, Makuuchi M, Takayama T, Imamura H, Kaneko J. Right lateral sector graft in adult living-related liver transplantation. Transplantation. 2002; 73:111–4.
13). Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmuller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003; 9:612–20.
Go to : 
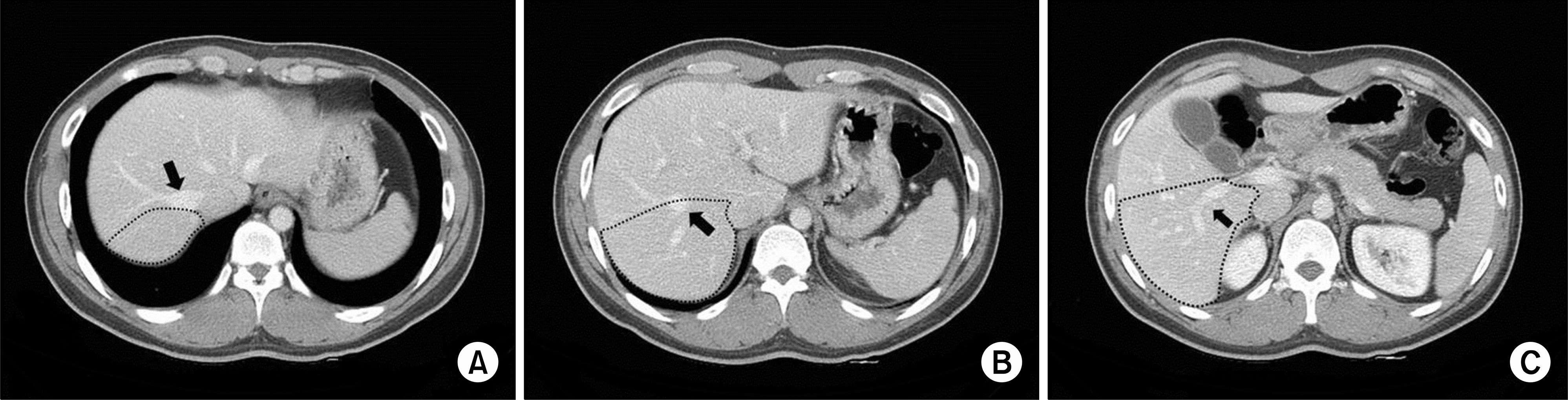 | Fig. 1.Right posterior sector (RPS) graft contours (dots) on axial computed tomography images. (A) Right hepatic vein (RHV) was the key border of RPS graft (arrow), (B) RHV (arrow), (C) Right posterior portal vein (arrow). |
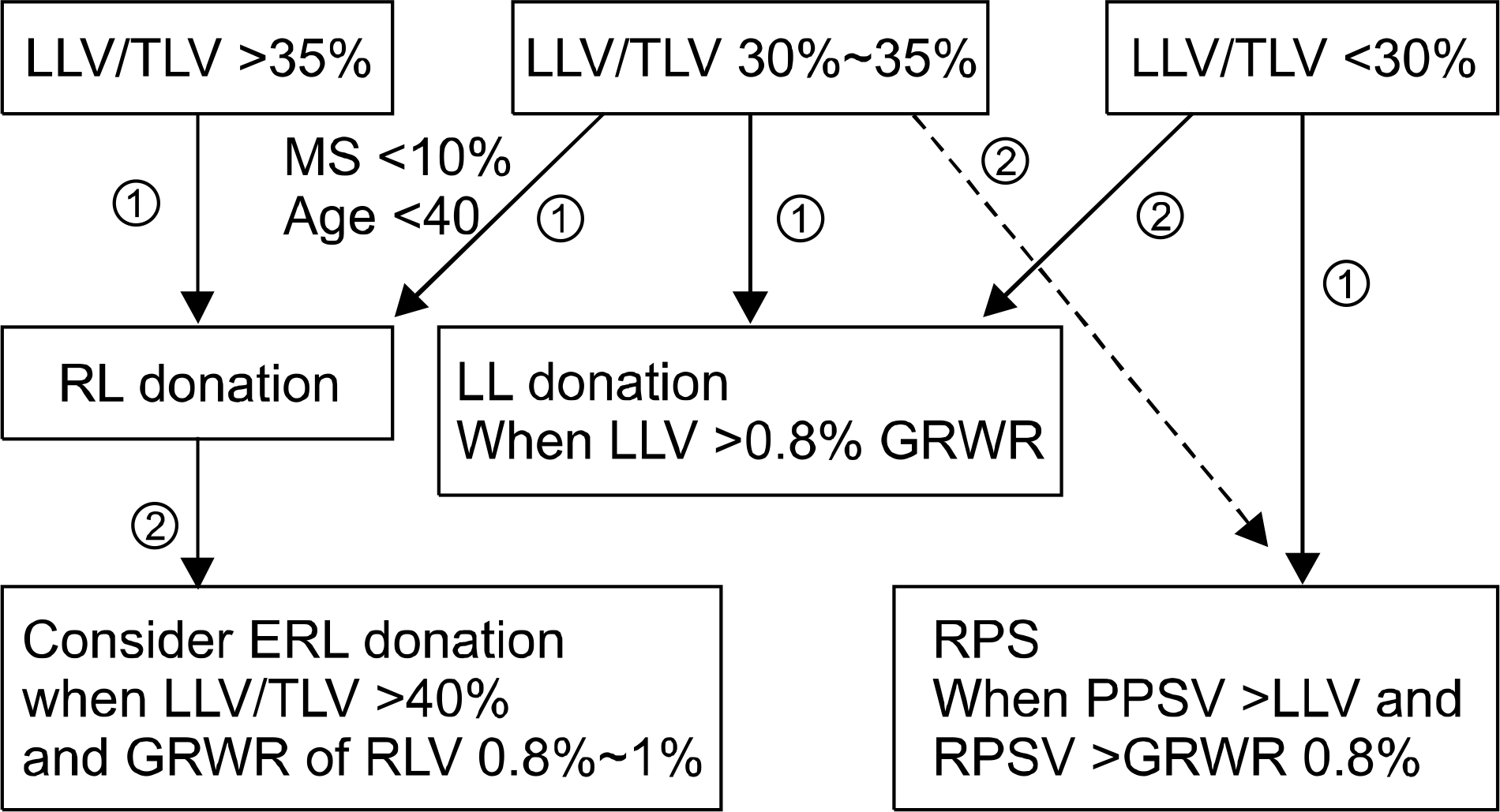 | Fig. 2.The flow chart of graft selection in the adult living donor liver transplantation. The graft was selected by the ratio of left lode volume (LLV)/total liver volume (TLV). The right lobe (RL) or extended right lode (ERL) donation was considered when the LLV/TLV greater than 35%. The left lobe (LL) donation was considered when the LLV/TLV less than 35% and LLV >0.8% of graft to recipient body weight ratio (GRWR). The right posterior sector (RPS) graft donation was primarily considered when LLV/TLV less than 30%, right posterior sector volume (RPSV) was greater than LLV, and RPSV >0.8% of GRWR. Also, the RPS graft was considered when RPSV > LLV among the candidate with 30%∼35% of LLV/TLV (dashed line). ① Primary consideration. ② Secondary consideration. Abbreviation: MS, macrovesicular steatosis. |
 | Fig. 3.A operation field of RPS graft procurement. (A) Hilar dissection for identifying the right posterior hepatic artery (arrow) and portal vein (arrowhead). (B) The surface marking for parenchymal transection was drawn along the ischemic demarcation. (C) A radiopaque marker for intraoperative cholangiography was located after parenchymal transection. (D) Wide graft outflow with a venous patch was made in the back-table. |
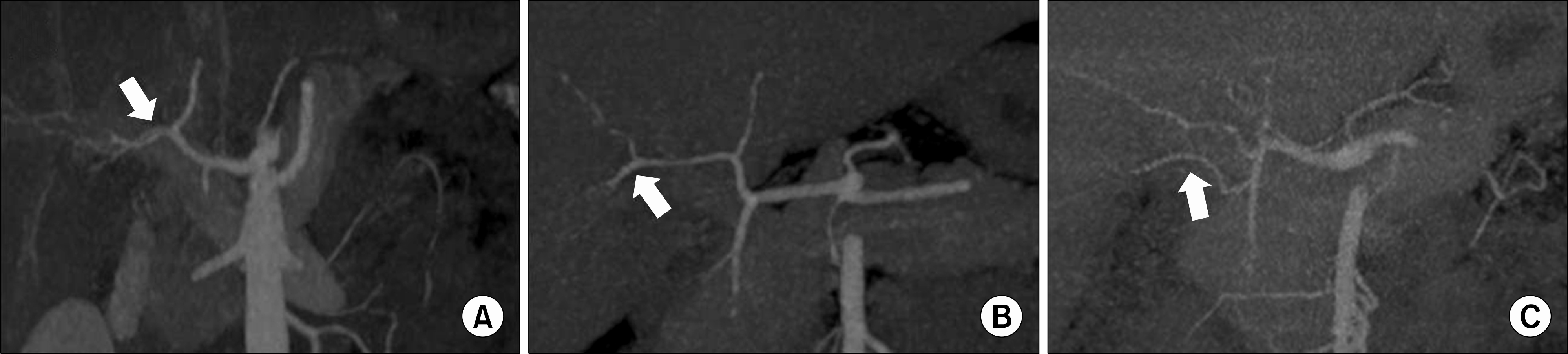 | Fig. 4.Anatomical right posterior hepatic artery (PHA) variations according to preoperative three-dimensional computed tomography. (A) The right PHA was early branching (EB) from right hepatic artery (HA), (B) The right PHA was late branching from right HA, (C) The right PHA was branching from gastroduodenal artery. |
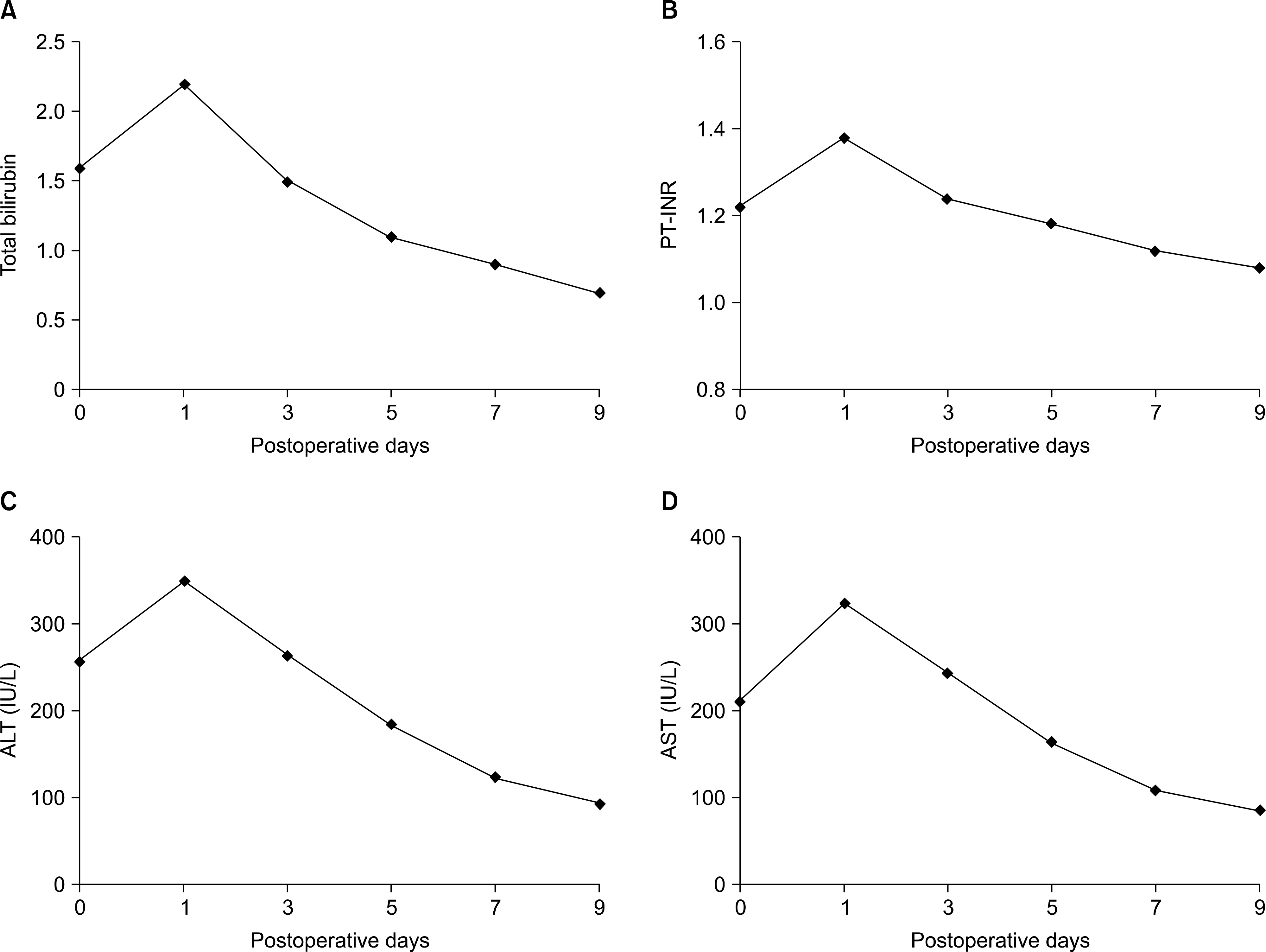 | Fig. 5.The mean value of the post-hepatectomy serum laboratory tests for the donors. (A) Total bilirubin, (B) prothrombin time-international normalized ratio (PT-INR), (C) alanine transaminase (ALT), (D) aspartate aminotransferase (AST). |
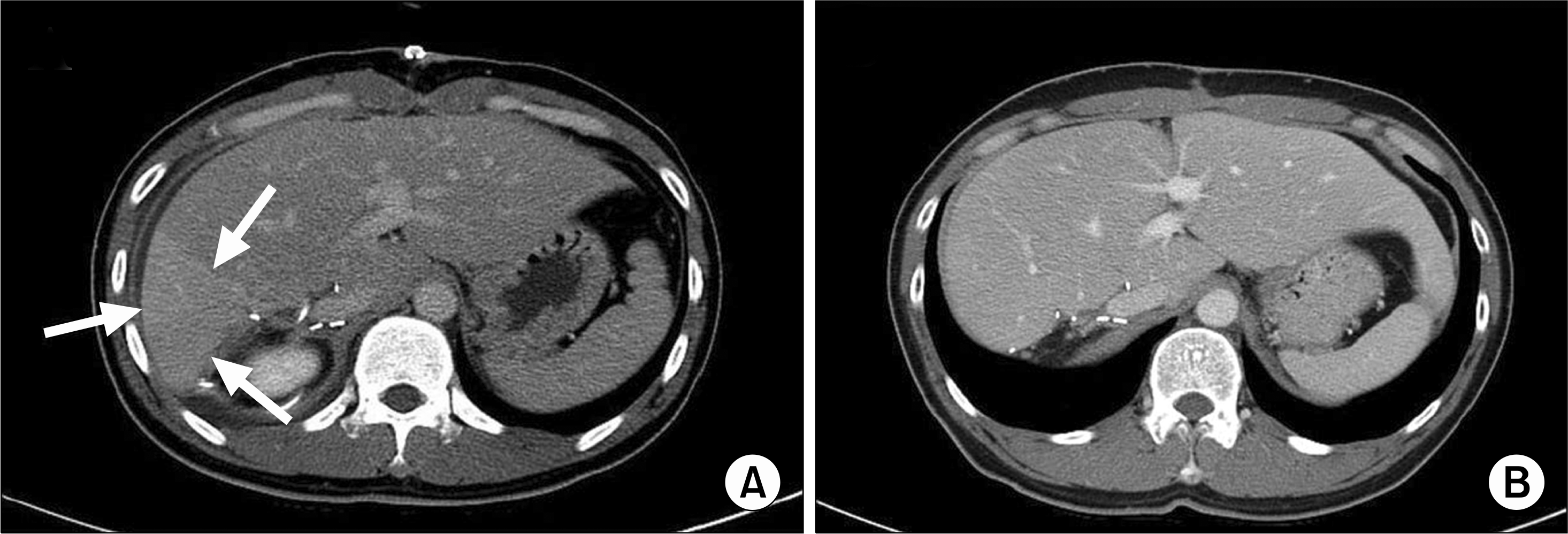 | Fig. 6.Postoperative computed tomography (CT) scans of a donor. (A) A CT scan on postoperative day 7 showed a congested area (arrows) in the remnant liver, (B) Three months after the operation, congested area disappeared and regeneration of remnant liver was seen. |
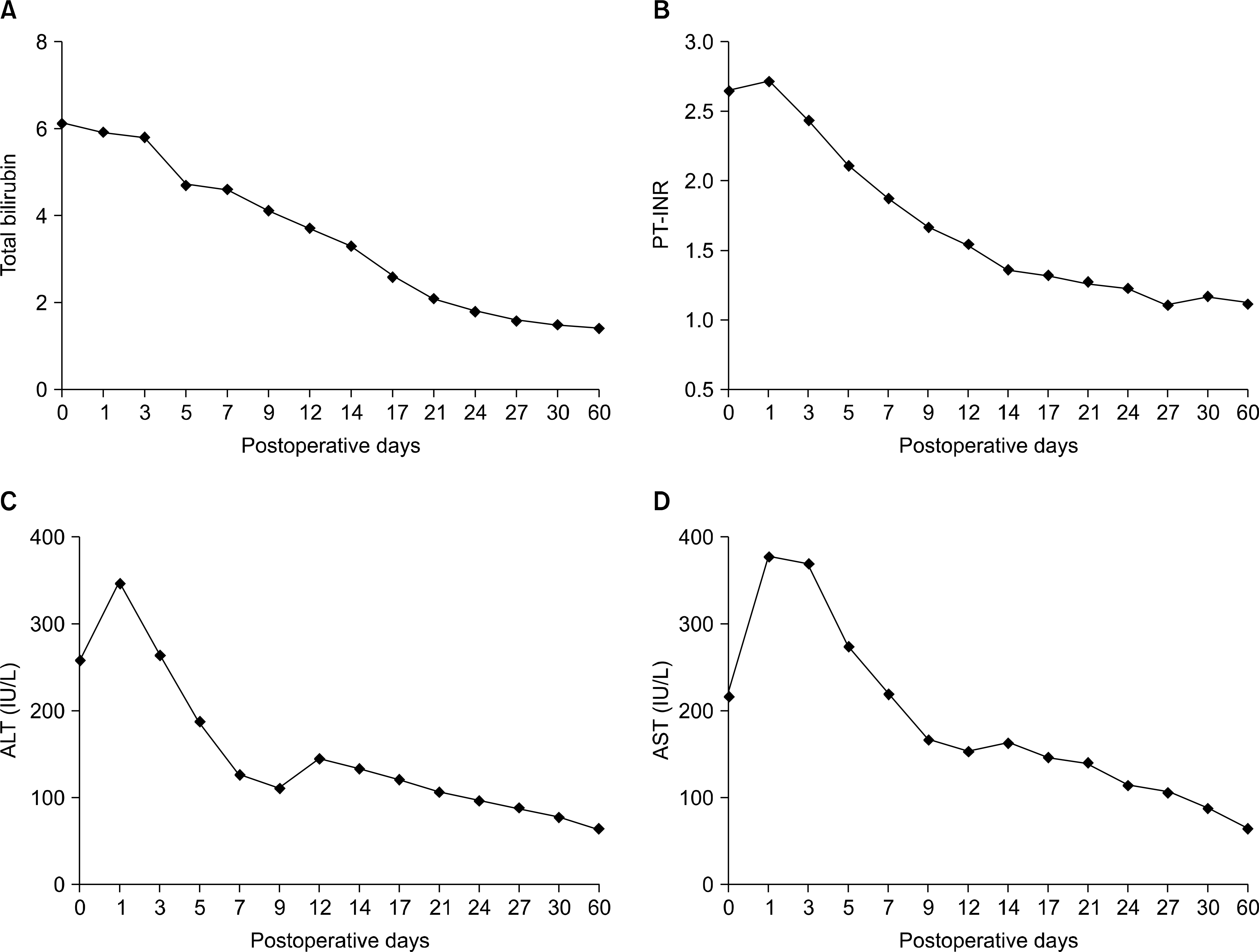 | Fig. 7.The mean value of the post-transplant serum laboratory tests for the recipients. (A) Total bilirubin, (B) prothrombin time-international normalized ratio (PT-INR), (C) alanine transaminase (ALT), (D) aspartate aminotransferase (AST). |
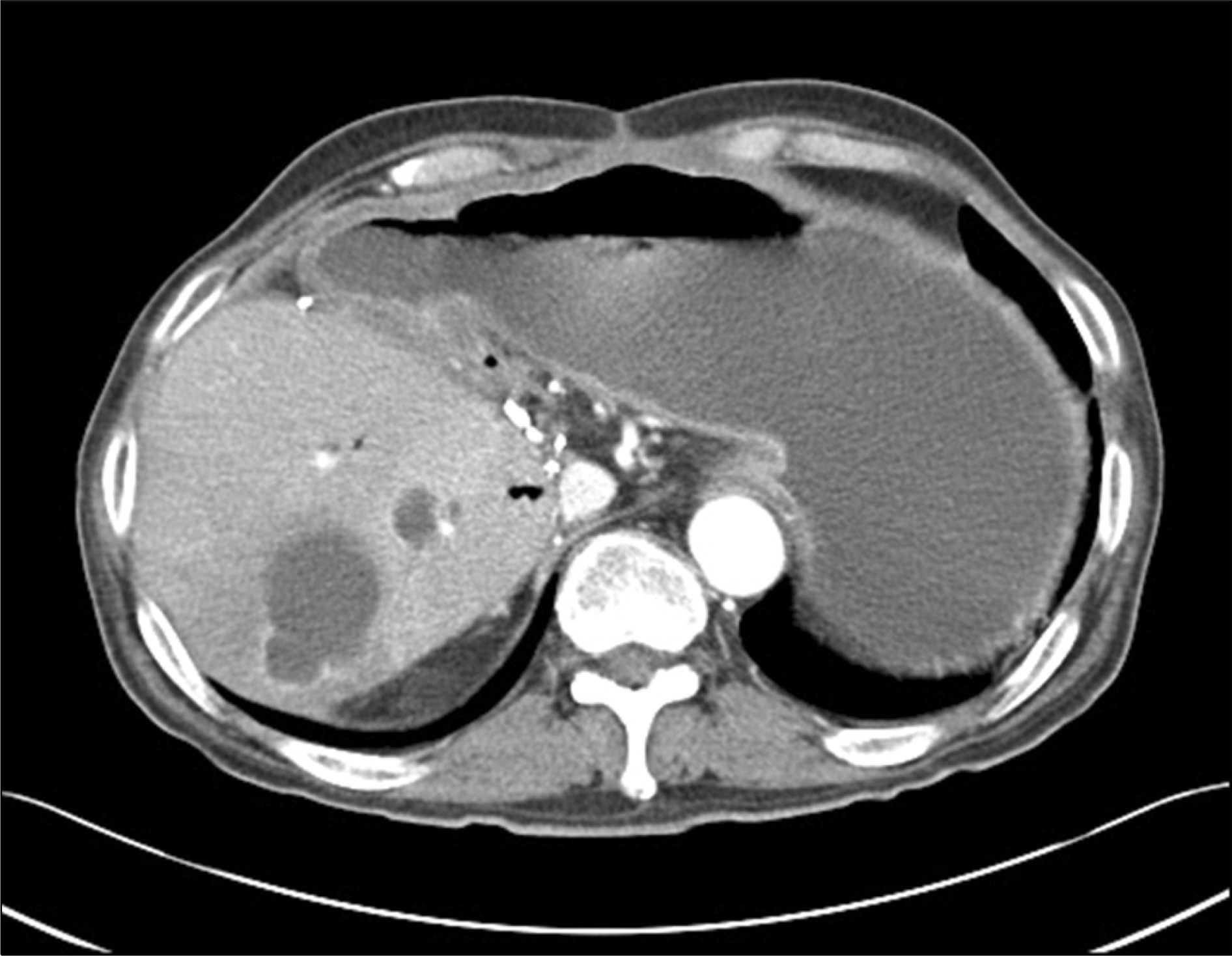 | Fig. 8.Severe liver abscess was developed in the right posterior sector graft in case 13th recipient who had performed Roux-en-Y hepaticojejunostomy. |
Table 1.
Recipients and donors’ profiles of the right posterior sector graft living donor liver transplantation
Table 2.
Operative measurements of the right posterior sector graft
Table 3.
Clinical outcomes of the recipients after the right posterior sector graft living donor liver transplantation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download