Abstract
Background
Ischemia-reperfusion injury (IRI) is a major cause of early graft dysfunction after lung transplantation. The aim of this study was to assess the effects of N-acetylcystein (NAC) and epigallocatechin-3-gallate (EGCG) on IRI of rat lungs.
Methods
Sprague-Dawley rats were divided into four groups. Sham group (n=6) did not receive IRI. Rats in the control group (n=6), NAC group (n=6), and EGCG group (n=6) were treated with an intraperitoneal injection of normal saline, NAC, and EGCG, respectively, prior to IRI. In the latter three groups, IRI was induced by clamping the left pulmonary artery, vein, and main stem bronchus for a period of 60 minutes. After ischemia, reperfusion and ventilation of the lung was allowed for a period of 180 minutes. The expression levels of inducible nitric oxide synthase (iNOS), hemeoxygenase-1 (HO-1), AMP-activated protein kinase-α(AMPK), and caveolin-1 in lung tissues were evaluated by Western blot. The pathological findings and the extent of pulmonary edema after IRI were compared among the groups.
Results
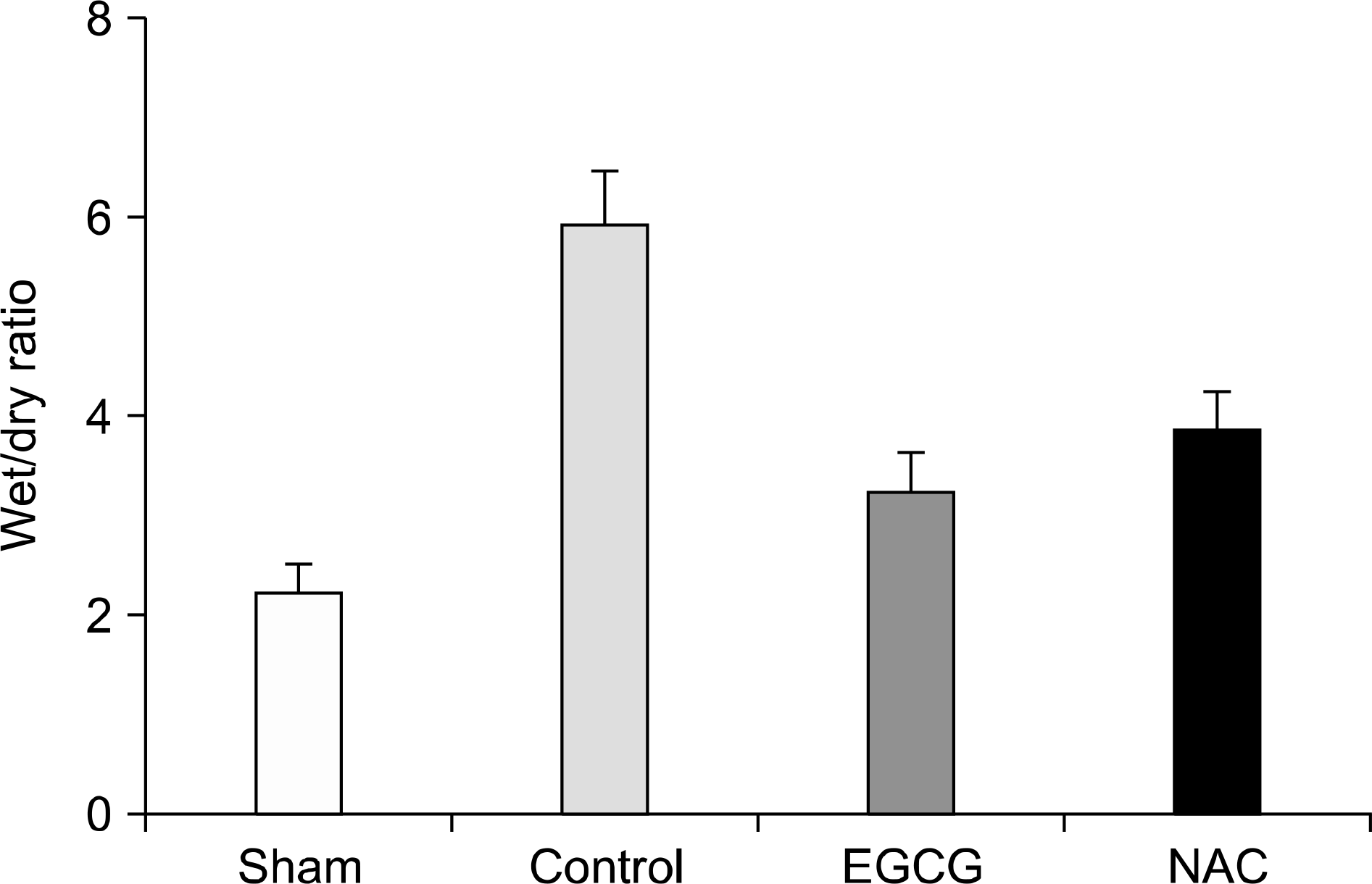
The expression levels of iNOS decreased in the Sham and EGCG groups. The expression level of HO-1 was significantly higher in the EGCG group (P=0.0001). Although the expression levels of AMPK and caveolin-1 showed no differences, the extent of phosphorylation of AMPK and caveolin-1 was higher in the EGCG and NAC groups, respectively. In hematoxylin-eosin staining, the lungs in the NAC and EGCG groups showed fewer alveolar injuries and less hemorrhagic congestion compared with the control group.
Go to : 
References
1). Mathias MA, Tribble CG, Dietz JF, Nguyen RP, Shockey KS, Kern JA, et al. Aprotinin improves pulmonary function during reperfusion in an isolated lung model. Ann Thorac Surg. 2000; 70:1671–4.

2). Kuwaki K, Komatsu K, Sohma H, Abe T. Improvement of ischaemia-reperfusion injury by lazaroid U74389G in rat lung transplantation model. Scand Cardiovasc J. 2000; 34:209–12.
3). Fischer S, Maclean AA, Liu M, Kalirai B, Keshavjee S. Inhibition of angiotensin-converting enzyme by captopril: a novel approach to reduce ischemia-reperfusion injury after lung transplantation. J Thorac Cardiovasc Surg. 2000; 120:573–80.

4). Kim JD, Baker CJ, Roberts RF, Darbinian SH, Marcus KA, Quardt SM, et al. Platelet activating factor acetylhydrolase decreases lung reperfusion injury. Ann Thorac Surg. 2000; 70:423–8.

5). Inci I, Dutly A, Rousson V, Boehler A, Weder W. Trimetazidine protects the energy status after ischemia and reduces reperfusion injury in a rat single lung transplant model. J Thorac Cardiovasc Surg. 2001; 122:1155–61.

6). Inci I, Inci D, Dutly A, Boehler A, Weder W. Melatonin attenuates posttransplant lung ischemia-reperfusion injury. Ann Thorac Surg. 2002; 73:220–5.

8). Arrick BA, Nathan CF. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984; 44:4224–32.
9). Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Ogletree ML, Brigham KL. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984; 73:1772–84.

10). Qiu Y, Bernier M, Hearse DJ. The influence of N-acetylcysteine on cardiac function and rhythm disorders during ischemia and reperfusion. Cardioscience. 1990; 1:65–74.
11). Giakoustidis AE, Giakoustidis DE, Iliadis S, Papageorgiou G, Koliakou K, Kontos N, et al. Attenuation of intestinal ischemia/reperfusion induced liver and lung injury by intraperitoneal administration of (−)-epigallocatechin-3-gallate. Free Radic Res. 2006; 40:103–10.

12). Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, et al. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004; 18:1621–3.
13). Gomez-Caro A, Garcia S, Reguart N, Cladellas E, Arguis P, Sanchez M, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central nonsmall cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg. 2011; 39:352–9.
14). Buttemeyer R, Philipp AW, Schlenzka L, Mall JW, Beissenhirtz M, Lisdat F. Epigallocatechin gallate can significantly decrease free oxygen radicals in the reperfusion injury in vivo. Transplant Proc. 2003; 35:3116–20.
15). Fang WF, Cho JH, He Q, Lin MC, Wu CC, Voelkel NF, et al. Lipid A fraction of LPS induces a discrete MAPK activation in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2007; 293:L336–44.

16). Welbourn CR, Goldman G, Paterson IS, Valeri CR, Shepro D, Hechtman HB. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg. 1991; 78:651–5.

17). Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med. 2013; 34:305–19.

18). Inci I, Zhai W, Arni S, Hillinger S, Vogt P, Weder W. N-ace-tylcysteine attenuates lung ischemia-reperfusion injury after lung transplantation. Ann Thorac Surg. 2007; 84:240–6.

19). Weinbroum AA, Kluger Y, Ben Abraham R, Shapira I, Karchevski E, Rudick V. Lung preconditioning with N-ace-tyl-L-cysteine prevents reperfusion injury after liver no flow-reflow: a doseresponse study. Transplantation. 2001; 71:300–6.
20). Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med. 1991; 91(3C):54S–9S.

21). Hulten LM, Lindmark H, Schersten H, Wiklund O, Nilsson FN, Riise GC. Butylated hydroxytoluene and N-acetylcysteine attenuates tumor necrosis factoralpha (TNF-alpha) secretion and TNF-alpha mRNA expression in alveolar macrophages from human lung transplant recipients in vitro. Transplantation. 1998; 66:364–9.
22). Chen L, Zhang HY. Cancer preventive mechanisms of the green tea polyphenol (−)-epigallocatechin-3-gallate. Molecules. 2007; 12:946–57.
23). Ignatov S, Shishniashvili D, Ge B, Scheller FW, Lisdat F. Amperometric biosensor based on a functionalized gold electrode for the detection of antioxidants. Biosens Bioele-ctron. 2002; 17:191–9.

24). Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998; 95:2175–9.

25). Phelps DT, Ferro TJ, Higgins PJ, Shankar R, Parker DM, Johnson A. TNF-alpha induces peroxynitrite-mediated depletion of lung endothelial glutathione via protein kinase C. Am J Physiol. 1995; 269(4 Pt 1):L551–9.

27). Lee PJ, Camhi SL, Chin BY, Alam J, Choi AM. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L175–82.

28). Soncul H, Oz E, Kalaycioglu S. Role of ischemic preconditioning on ischemia-reperfusion injury of the lung. Chest. 1999; 115:1672–7.

29). Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, et al. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002; 73:287–92.
30). Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, et al. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001; 1:121–8.

31). Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999; 277(1 Pt 1):E1–10.
32). Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol. 2013; 182:1021–30.
33). Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, et al. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007; 282:13736–45.

34). Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-acti-vated protein kinase, Akt, and FOXO1. Endocrinology. 2010; 151:103–14.

35). Young LH, Ikeda Y, Lefer AM. Caveolin-1 peptide exerts cardioprotective effects in myocardial ischemia-reperfusion via nitric oxide mechanism. Am J Physiol Heart Circ Physiol. 2001; 280:H2489–95.

36). Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007; 21:1565–74.

Go to : 
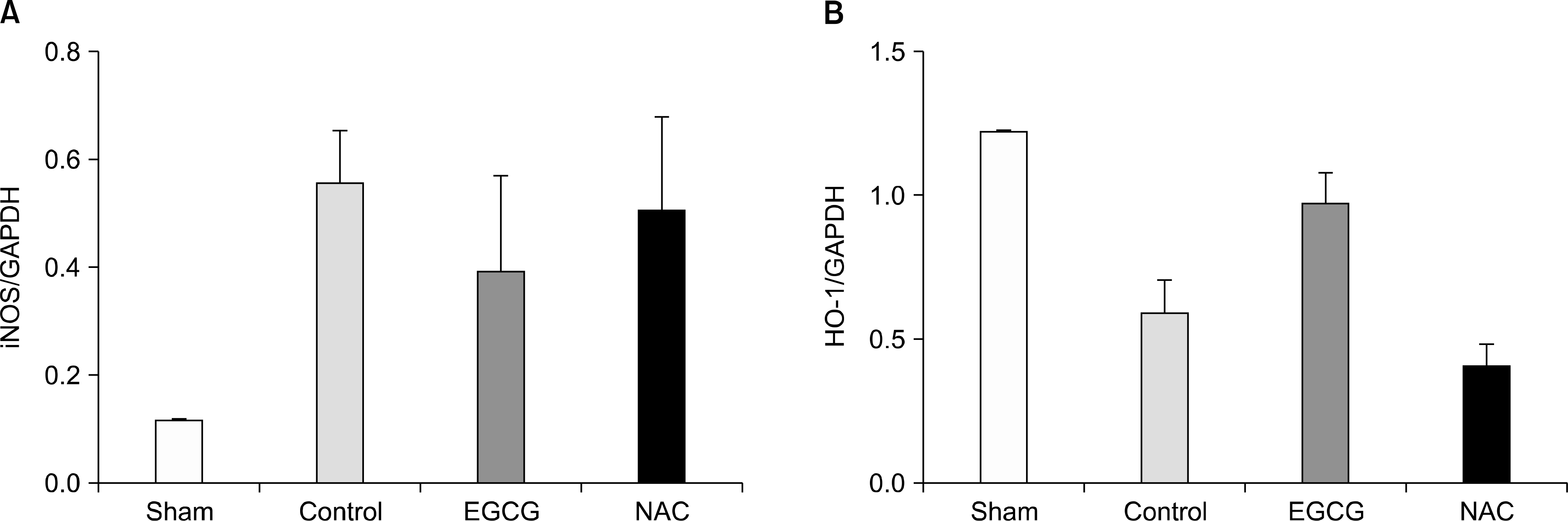 | Fig. 1.(A) The expression levels of inducible nitric oxide synthase (iNOS) in lung tissues. Sham and epigallocatechin-3-gallate (EGCG) group showed lower the expression levels but, these were not significant (P=0.477). (B) The expression levels of hemeoxygenase-1 (HO-1) in lung tissues. The expression levels of HO-1 had the significant differences (P=0.001). EGCG group showed the higher level than N-acetylcystein (NAC) group (P=0.005; n=6 in each group; mean±SD). Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
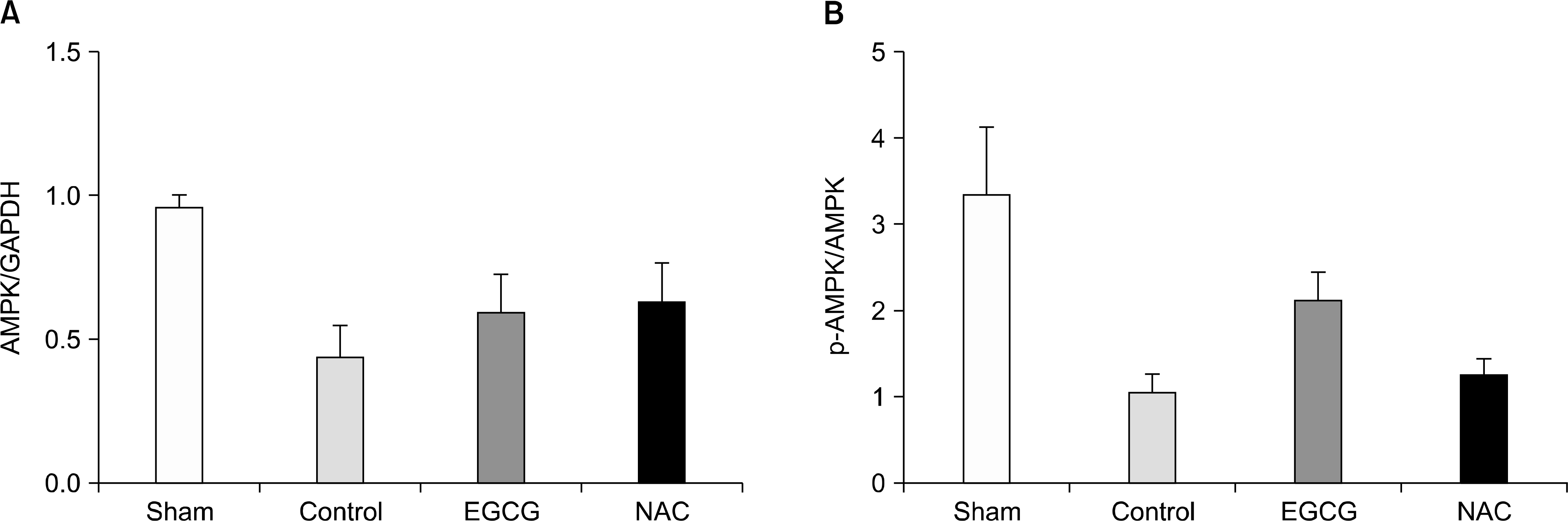 | Fig. 2.(A) The expression levels of AMP-activated protein kinase (AMPK) in lung tissues. There were no differences in the expression levels of AMPK. (B) The extents of phosphorylations of AMPK. The extents of phosphorylation in sham and epigallocatechin-3-gallate (EGCG) group were higher (P=0.002), but there was no difference between control and N-acetylcystein (NAC) group (P=1.000; n=6 in each group; mean±SD). Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
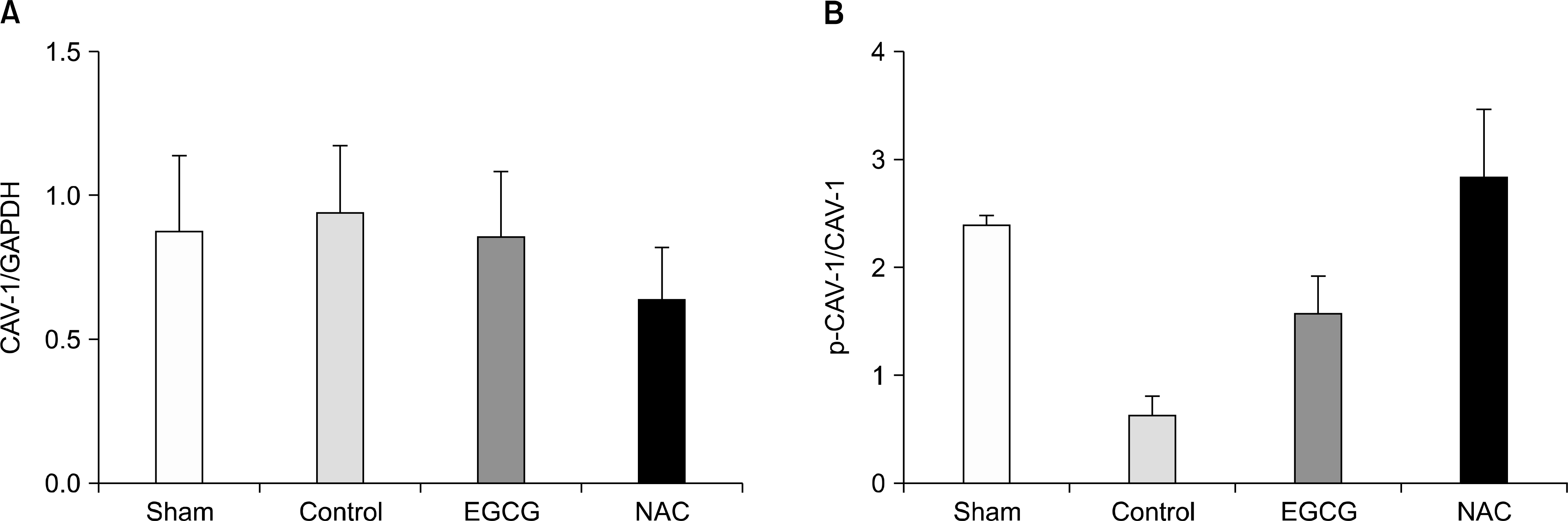 | Fig. 3.(A) The expression levels of caveolin-1 (CAV-1) in lung tissues. There were no differences in the expression levels of CAV-1 among groups (P=0.784). (B) The extents of phosphorylations of CAV-1. In N-acetylcystein (NAC) group, the extent of phosphorylation of CAV-1 was higher (P=0.010). Although epigallocatechin-3-gallate (EGCG) group showed the higher extent of phosphorylation than control group, it was not significant (P=0.753; n=6 in each group; mean±SD). Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
 | Fig. 4.Pathologic findings of lung after ischemia-reperfusion injury (HE stain, ×40). (A) Sham group: the alveolar structure was maintained well and there was no congestion. (B) Control group: the most of alveolar structure was destroyed and severe neutrophil infiltration and congestion were found. (C) Epigallocatechin-3-gallate (EGCG) group: although mild neutrophil infiltration and congestion were found, alveolar structure was maintained relatively well. (D) N-acet-ylcystein group: the finding was similar to that of EGCG group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download