Abstract
Background
Kidney transplantation is the most effective treatment in patients with chronic kidney disease. Recently, the survival rate of kidney allografts has been markedly increased by the development of immunosuppressants. According to research reports published in Symphony in 2007 and 2009, low dose tacrolimus/mycophenolate mofetil (MMF) showed better results than cyclosporin/MMF in renal function and rejection.
Methods
We compared patient survival rate, graft survival rate, incidence of rejection, and metabolic complications in two groups of patients who received immunosuppressants with either tacrolimus/MMF/steroid or cyclosporin/MMF/steroid. All patients underwent kidney transplants at Keimyung University Dongsan Medical Center between January 1997 and December 2003 with follow-up over 10 years.
Results
A total of 180 patients were included in the research (117 patients were treated with tacrolimus/MMF/steroid and 63 patients with cyclosporin/MMF/steroid). The incidence rate of acute rejection was higher in the cyclosporin/MMF/steroid group; however, the difference was not statistically significant. In the case of metabolic complications, new onset diabetes after transplantation was more frequent in the tacrolimus/MMF/steroid group. The cyclosporin/MMF/steroid group appeared to have a higher rate of hypertension and hyperlipidemia.
Go to : 
References
2). Djamali A, Premasathian N, Pirsch JD. Outcomes in kidney transplantation. Semin Nephrol. 2003; 23:306–16.

3). Landsberg DN, Shapiro J. Kidney, pancreas, and pancreatic islet transplantation. BC Med J. 2010; 52:189–96.
4). Han SY, Kim YR, Jang MH, Hwang EA, Park SB, Park UJ. Long-term clinical outcomes of kidney transplantation from 1982 to 2013 at Keimyung University Dongsan Medical Center of Korea [abstract]. The 13th Congress of the Asian Society of Transplantation; 2013 Sep 2–6; Kyoto, Japan. [place unknown]: Asian Society of Transplantation;. 2013.
5). Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007; 357:2562–75.

6). Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009; 9:1876–85.

7). Chapter 2: initial maintenance immunosuppressive medications. Am J Transplant. 2009; 9(Suppl 3):S10–3.
8). Kim YS, Kim SI, Kim MS, Huh KH, Ju MK, Joo DJ, et al. Evaluation of independent risk factors affecting renal allograft survival by transplant era. J Korean Soc Transplant. 2012; 26:178–87.

9). Fukuhara N, Ono Y, Kinukawa T, Hattori R, Nishiyama N, Yamada S, et al. Long-term outcome of tacrolimus in cadaveric kidney transplantation from non-heart-beating donors. Transplant Proc. 2002; 34:1577–9.

10). Sandrini S, Aslam N, Tardanico R, Setti G, Bossini N, Valerio F, et al. Tacrolimus versus cyclosporine for early steroid withdrawal after renal transplantation. J Nephrol. 2012; 25:43–9.

11). Kim HC, Hwang EA, Han SY, Park SB, Kim HT, Cho WH. Primary immunosuppression with tacrolimus in kidney transplantation: three-year follow-up in a single center. Transplant Proc. 2004; 36:2082–3.

12). Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003; 349:2326–33.

13). Evenepoel P, Vanrenterghem Y. Death with functioning graft: a preventable cause of graft loss. Ann Transplant. 2001; 6:17–20.
14). Sato K, Tadokoro F, Ishida K, Matsuzawa K, Nakayama Y, Yokota K, et al. Causes of death after renal transplantation: a longterm follow-up study. Transplant Proc. 1994; 26:2017–8.
15). Hiesse C, Rieu P, Larue JR, Kriaa F, Goupy C, Benoit G, et al. Late graft failure and death in renal transplant recipients: analysis in a singlecenter population of 1500 patients. Transplant Proc. 1997; 29:240–2.

16). Hwang E, Jang M, Kwak C, Han S, Park S, Kim H, et al. The changes of graft survival and causes of graft failure after kidney transplantation. J Korean Soc Transplant. 2011; 25:22–30.

17). Boots JM, van Duijnhoven EM, Christiaans MH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in renal transplant recipients on tacrolimus: the effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol. 2002; 13:221–7.

18). Hecking M, Kainz A, Werzowa J, Haidinger M, Döller D, Tura A, et al. Glucose metabolism after renal transplantation. Diabetes Care. 2013; 36:2763–71.

19). van Duijnhoven EM, Christiaans MH, Boots JM, Nieman FH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol. 2002; 13:213–20.

21). Margreiter R. European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet. 2002; 359:741–6.

22). Luft FC. How calcineurin inhibitors cause hypertension. Nephrol Dial Transplant. 2012; 27:473–5.

23). Pirsch JD. Cytomegalovirus infection and posttransplant lymphoproliferative disease in renal transplant recipients: results of the U.S. multicenter FK506 Kidney Transplant Study Group. Transplantation. 1999; 68:1203–5.

24). Lindholm A, Ohlman S, Albrechtsen D, Tufveson G, Persson H, Persson NH. The impact of acute rejection episodes on longterm graft function and outcome in 1347 primary renal transplants treated by 3 cyclosporine regimens. Transplantation. 1993; 56:307–15.

25). Almond PS, Gillingham KJ, Sibley R, Moss A, Melin M, Leventhal J, et al. Renal transplant function after ten years of cyclosporine. Transplantation. 1992; 53:316–23.

26). Opelz G, Mytilineos J, Scherer S, Schwarz V. Clinical implications of DNA typing in organ transplantation. The Collaborative Transplant Study. Transplant Proc. 1997; 29:1524–7.
27). Gulanikar AC, MacDonald AS, Sungurtekin U, Belitsky P. The incidence and impact of early rejection episodes on graft outcome in recipients of first cadaver kidney transplants. Transplantation. 1992; 53:323–8.

28). Tanabe K, Ishikawa N, Tokumoto T, Takahashi K, Oshima T, Fuchinoue S, et al. Factors affecting long-term renal allograft survival in cyclosporine-treated kidney transplants. Transplant Proc. 1998; 30:1805–9.

29). Meng HL, Jin XB, Li XT, Wang HW, Lü JJ. Impact of human leukocyte antigen matching and recipients' panel reactive antibodies on two-year outcome in presensitized renal allograft recipients. Chin Med J (Engl). 2009; 122:420–6.
30). Susal C, Opelz G. Kidney graft failure and presensitization against HLA class I and class II antigens. Transplantation. 2002; 73:1269–73.
31). Kim JY, Kim SH, Kim YS, Choi BS, Kim JC, Park SC, et al. Report of 1,500 kidney transplants at the Catholic University of Korea. J Korean Soc Transplant. 2006; 20:172–80.
32). Moon JI, Lee CM, Kim SI, Kim MS, Kim YS, Park K. The impact of acute rejection on longterm graft outcome in renal allograft recipient. J Korean Soc Transplant. 1998; 12:67–74.
33). Jung HT, Jung GO, Choi GS, Kwon CH, Kim SJ, Joh JW, et al. Report of 1,000 kidney transplants at the Sungkyunkwan University of Korea. J Korean Soc Transplant. 2009; 23:141–8.

Go to : 
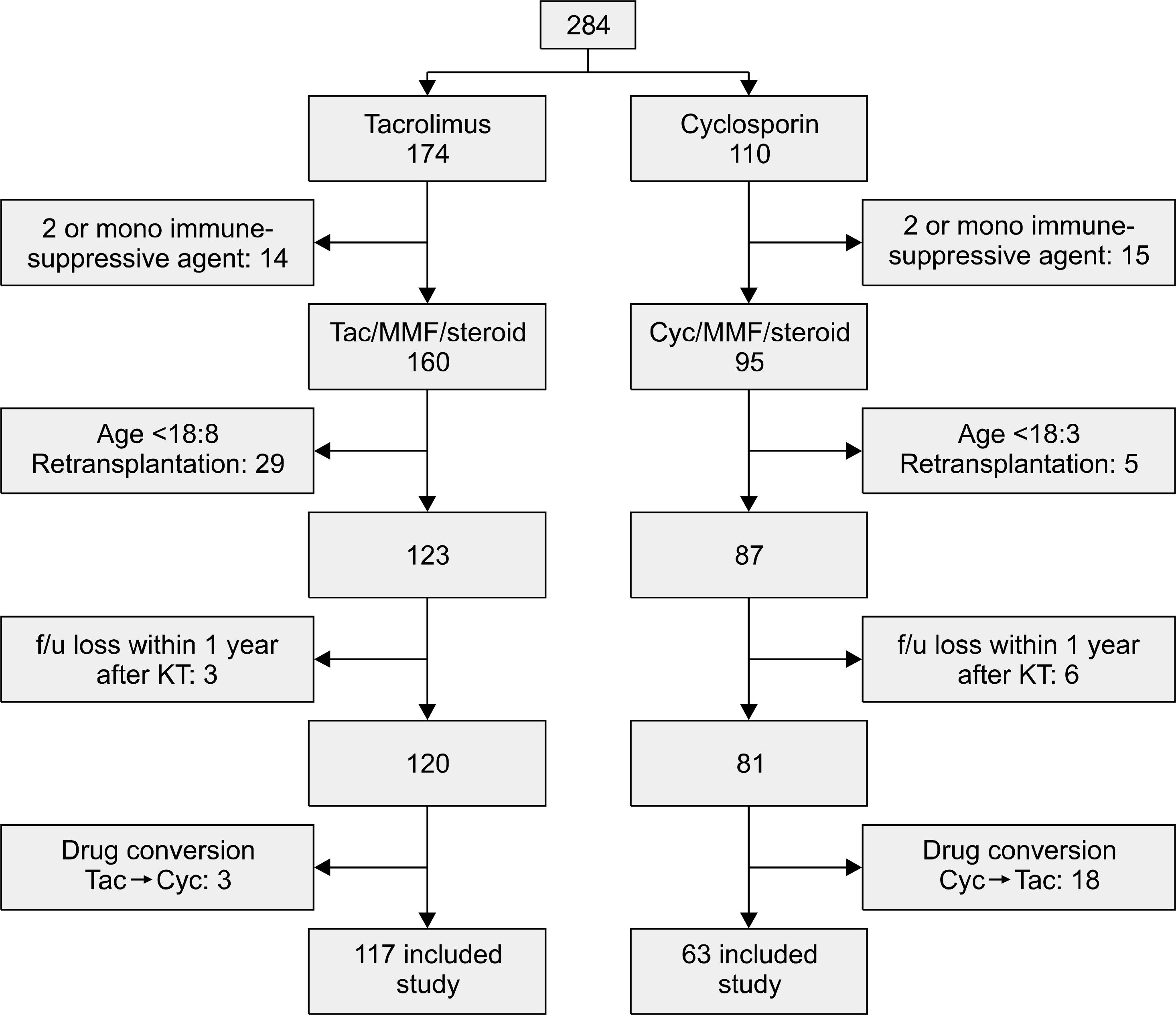 | Fig. 1.The flow diagram shows patient selection process, with inclusion and exclusion criteria. Abbreviations: Tac, tacrolimus; MMF, mycophenolate mofetil; Cyc, cyclosporine; f/u, follow-up; KT, kidney transplantation. |
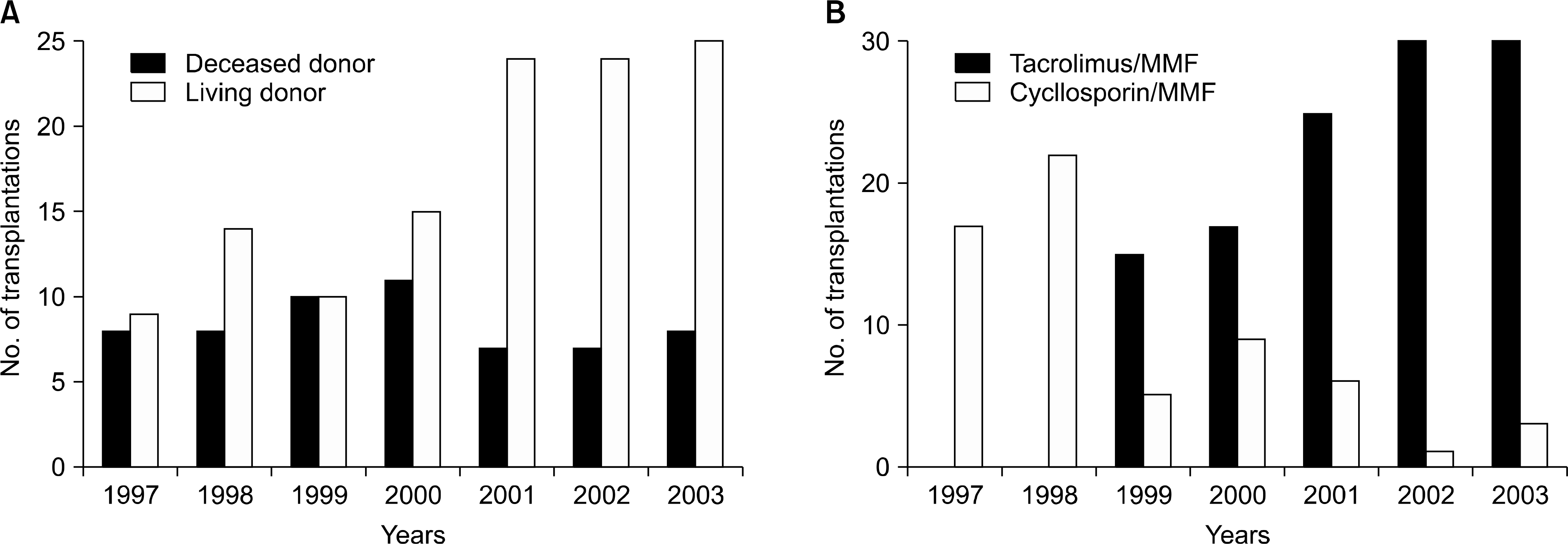 | Fig. 2.The annual changes of donor type (A) and immunosuppressive agent (B) in kidney transplantation from 1997 to 2003. Abbreviation: MMF, mycophenolate mofetil. |
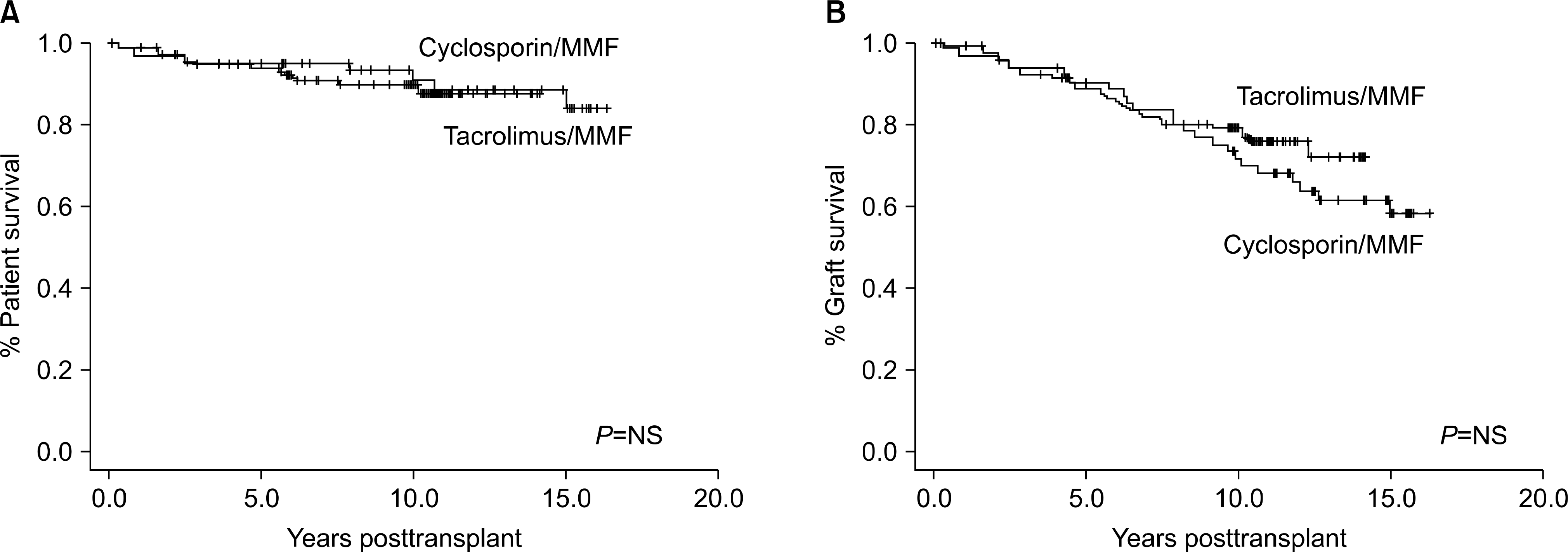 | Fig. 3.Patient (A) and graft (B) survival of kidney transplantation according to maintenance immunosuppressant. Abbreviations: MMF, mycophenolate mofetil; NS, not significant. |
Table 1.
The baseline characteristics of the study population
Table 2.
The incidence of acute rejection and graft loss
Table 3.
The incidence of complications
Table 4.
The incidence of infection events
Table 5.
The comparison of characteristics between graft loss and survival group
Table 6.
Univariated and multivariated analysis for risk factors of graft loss




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download