Abstract
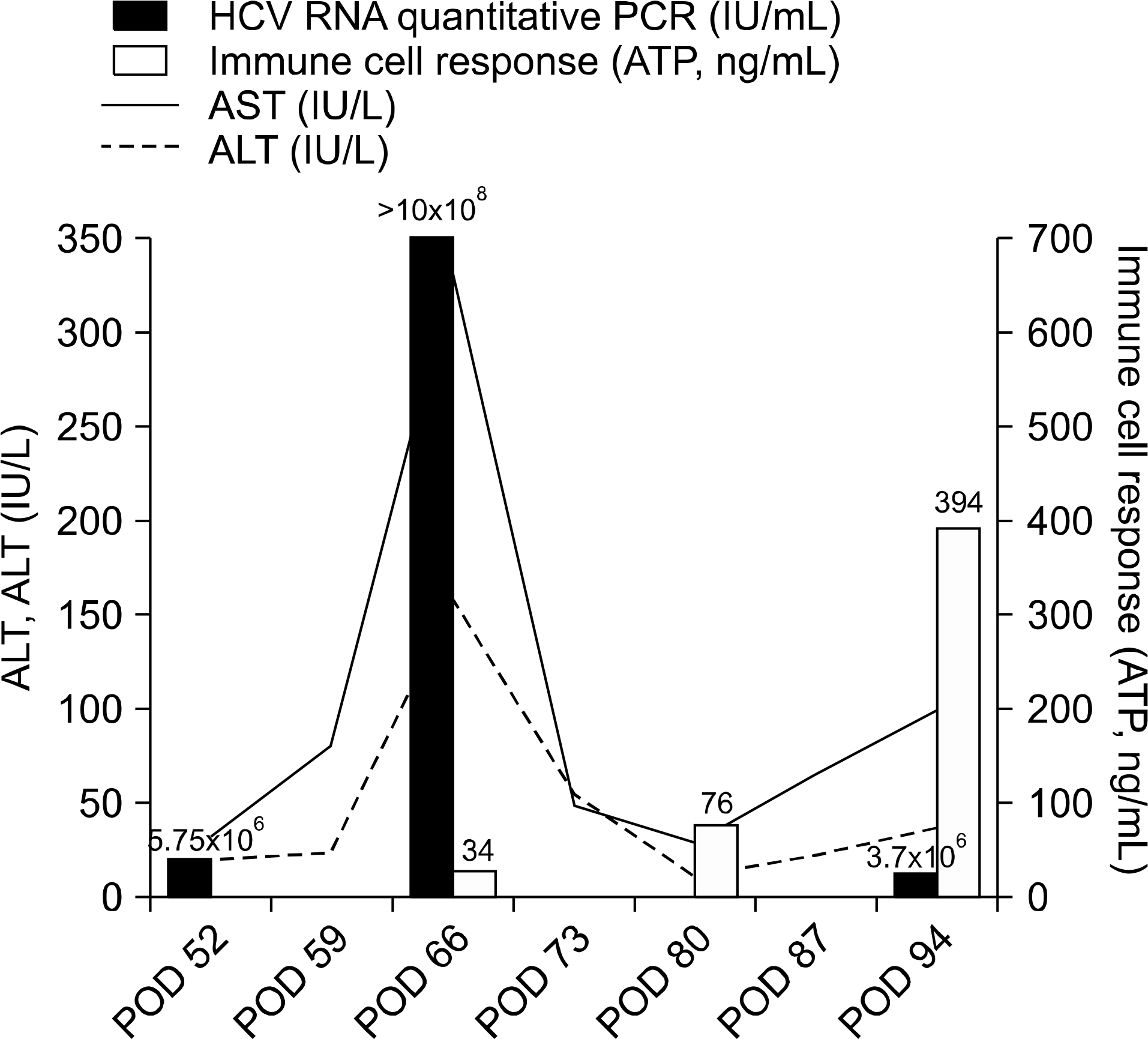
We report on the usefulness of the ImmuKnow assay in a case of suspected acute cellular rejection after liver transplantation. A 58-year-old male with hepatocellular carcinoma and liver cirrhosis caused by chronic hepatitis C had undergone tumorectomy 2 months previously. Following surgery, the underlying cirrhosis and hepatic encephalopathy were aggravated. The patient had been listed for liver transplantation and underwent cadaveric donor liver transplantation. Approximately 11 days after discharge, the patient developed mild fever and diarrhea and was rehospitalized. A liver biopsy showed histologic features associated with cellular rejection. According to the histopathologic diagnosis, the dosage of tacrolimus was increased from 5 to 7 mg twice daily. After changing the dose, aspartate aminotransferase and alanine aminotransferase were elevated, findings not corresponding to the former diagnosis. Hepatitis C virus (HCV) quantitative assay and ImmuKnow assay were performed for further evaluation. High HCV viral load and a very low ATP level detected using the ImmuKnow assay were suggestive of recurrent HCV rather than acute cellular rejection. Two weeks after reducing the immunosuppressant dosage and treating with antiviral therapy using ribavirin, the patient showed clinical improvement with a decrease in HCV viral load and a normal ATP level. Due to overlapping histologic features, acute cellular rejection can be difficult to distinguish from recurrent HCV. As in this case, the ATP level detected using the ImmuKnow assay is considered a reliable marker of cellular immune status. Immune monitoring of transplant patients may assist in making a differential diagnosis and in minimizing the adverse events of immunosuppression.
References
1). Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998; 28:638–45.

2). Choi YR, Lee KW. The management of HCV recurrence after liver transplantation. J Korean Soc Transplant. 2013; 27:37–41. (최영록, 이광웅. 간이식 후 HCV 치료 및 면역억제제. 대한이식학회지 2013;27: 37–41.).

3). Petrovic LM, Villamil FG, Vierling JM, Makowka L, Geller SA. Comparison of histopathology in acute allograft rejection and recurrent hepatitis C infection after liver transplantation. Liver Transpl Surg. 1997; 3:398–406.

4). Regev A, Molina E, Moura R, Bejarano PA, Khaled A, Ruiz P, et al. Reliability of histopathologic assessment for the differentiation of recurrent hepatitis C from acute rejection after liver transplantation. Liver Transpl. 2004; 10:1233–9.

5). Alkhouri N, Hanouneh IA, Lopez R, Zein NN. Monitoring peripheral blood CD4+ adenosine triphosphate activity in recurrent hepatitis C and its correlation to fibrosis progression. Liver Transpl. 2010; 16:155–62.

6). Cabrera R, Ararat M, Soldevila-Pico C, Dixon L, Pan JJ, Firpi R, et al. Using an immune functional assay to differentiate acute cellular rejection from recurrent hepatitis C in liver transplant patients. Liver Transpl. 2009; 15:216–22.

7). Israeli M, Klein T, Sredni B, Avitzur Y, Mor E, Bar-Nathen N, et al. ImmuKnow: a new parameter in immune monitoring of pediatric liver transplantation recipients. Liver Transpl. 2008; 14:893–8.

8). Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, et al. Assessing relative risks of infection and rejection: a metaanalysis using an immune function assay. Transplantation. 2006; 82:663–8.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download