Abstract
Tuberous sclerosis complex (TSC) is a neurocutaneous disease characterized by the formation of hamartomas in multiple organs. TSC can show lesions including facial angiofibroma, shagreen patch on the skin, cortical tuber, subependymal nodule, astrocytoma in the brain, cardiac rhabdomyoma, and renal angiomyolipoma. In particular, renal angiomyolipoma may be a cause of end-stage renal disease (ESRD). On the other hand, sirolimus has regulatory effects on cellular growth and proliferation via its inhibitory effect on a protein, mammalian target of rapamycin. We report on a case of an 18-year-old male who underwent renal transplantation due to ESRD induced by TSC. Sirolimus played a role in successful treatment of TSC and effective immunosuppression for transplantation.
References
1). Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006; 355:1345–56.

2). Rakowski SK, Winterkorn EB, Paul E, Steele DJ, Halpern EF, Thiele EA. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int. 2006; 70:1777–82.

3). Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease: a contiguous gene syndrome. Nat Genet. 1994; 8:328–32.
4). Lim CC, Tan H, Thangaraju S, Lai AH, Foo MW. End-stage renal disease in tuberous sclerosis complex-polycystic kidney disease contiguous gene syndrome: epidemiology, clinical manifestations and implications for transplantation. Int Urol Nephrol. 2014; 46:1869–70.

5). Koh CY, Cho YS, Kim HJ, Kim JW, Jun DH. Angiomyolipoma rupture and spontaneous pneumothorax in tuberous sclerosis patients: a case report. J Korean Soc Emerg Med. 2012; 23:734–7. (고찬영, 조영순, 김호중, 김재우, 전덕호. 결절성경화증 환자에게 동반된 혈관근지방종 파열과 자발성 기흉 1례. 대한응급의학회지 2012;23: 734–7.).
6). Park SH, Son JW, Park CK, Park MJ, Yoo JH, Kang HM. A case of pulmonary lymphangioleiomyomatosis associated with tuberous sclerosis and renal angiomyolipoma. Korean J Med. 2011; 81:775–9. (박소희, 손주웅, 박철기, 박명재, 유지홍, 강홍모. 신장을 침범한 결정성 경화증에서 발생한 폐의 임파관평활근종증 1예. 대한내과학회지 2011;81: 775–9.).
7). Lee KH, Lee N, Lee CK, Hong SM, Seong GJ, Kim CY. Retinal nerve fiber layer defect associated with astrocytic hamartoma in a patient with tuberous sclerosis. J Korean Ophthalmol Soc. 2013; 54:1282–6. (이가현, 이나은, 이창규, 홍사민, 성공제, 김찬윤. 결절경화증환자에서 발생한 성상세포 과오종과 연관된 시신경섬유 결손. 대한안과학회지 2013;54: 1282–6.).

8). Gó mez MR. History of the tuberous sclerosis complex. Brain Dev. 1995; 17(Suppl):55–7.
9). Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Hum Genet. 2000; 107:97–114.

10). Shepherd CW, Gomez MR, Lie JT, Crowson CS. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991; 66:792–6.

11). Feldman S, Libertino JA, Dowd JB. Hamartoma and renal transplant implications. J Urol. 1975; 114:460–2.

12). Balligand JL, Pirson Y, Squifflet JP, Cosyns JP, Alexandre GP, van Ypersele de Strihou C. Outcome of patients with tuberous sclerosis after renal transplantation. Transplantation. 1990; 49:515–8.

13). Rosado C, Garcí a-Cosmes P, Fraile P, Vá zquez-Sá nchez F. Tuberous sclerosis associated with polycystic kidney disease: effects of rapamycin after renal transplantation. Case Rep Transplant. 2013; 2013:397087.

14). Tarasewicz A, Debska-Slizień A, Konopa J, Zdrojewski Z, Rutkowski B. Rapamycin as a therapy of choice after renal transplantation in a patient with tuberous sclerosis complex. Transplant Proc. 2009; 41:3677–82.

15). Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006; 124:471–84.

16). Curatolo P, Moavero R. mTOR inhibitors in tuberous sclerosis complex. Curr Neuropharmacol. 2012; 10:404–15.

17). Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008; 358:140–51.

Fig. 1.
(A) Facial angiofibromas. Multiple erythematous papules with telangiectasia were located on his nose and cheeks on physical examination. (B) Shagreen patch on the left back. Flesh-colored soft plaques were seen on the left back. (C) Ungual fibroma on left third finger. Fibrous growth arising from nail bed was noted in patient's left third finger.
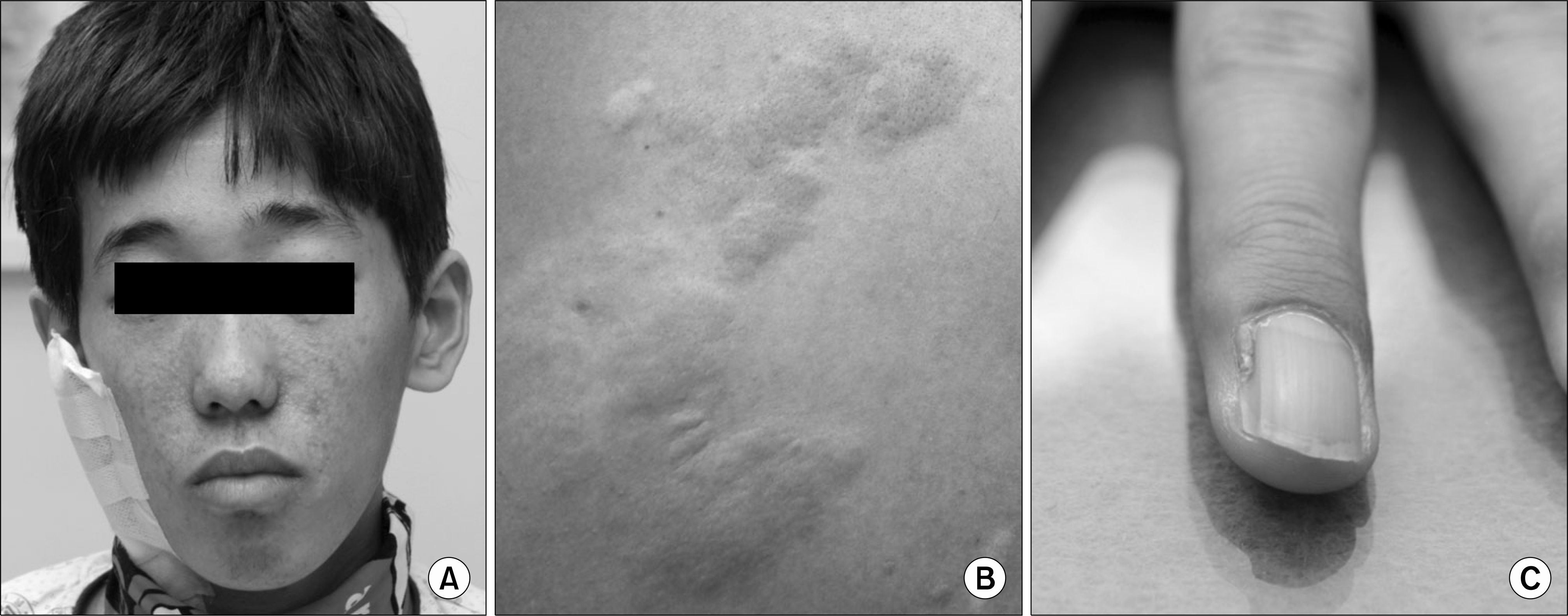
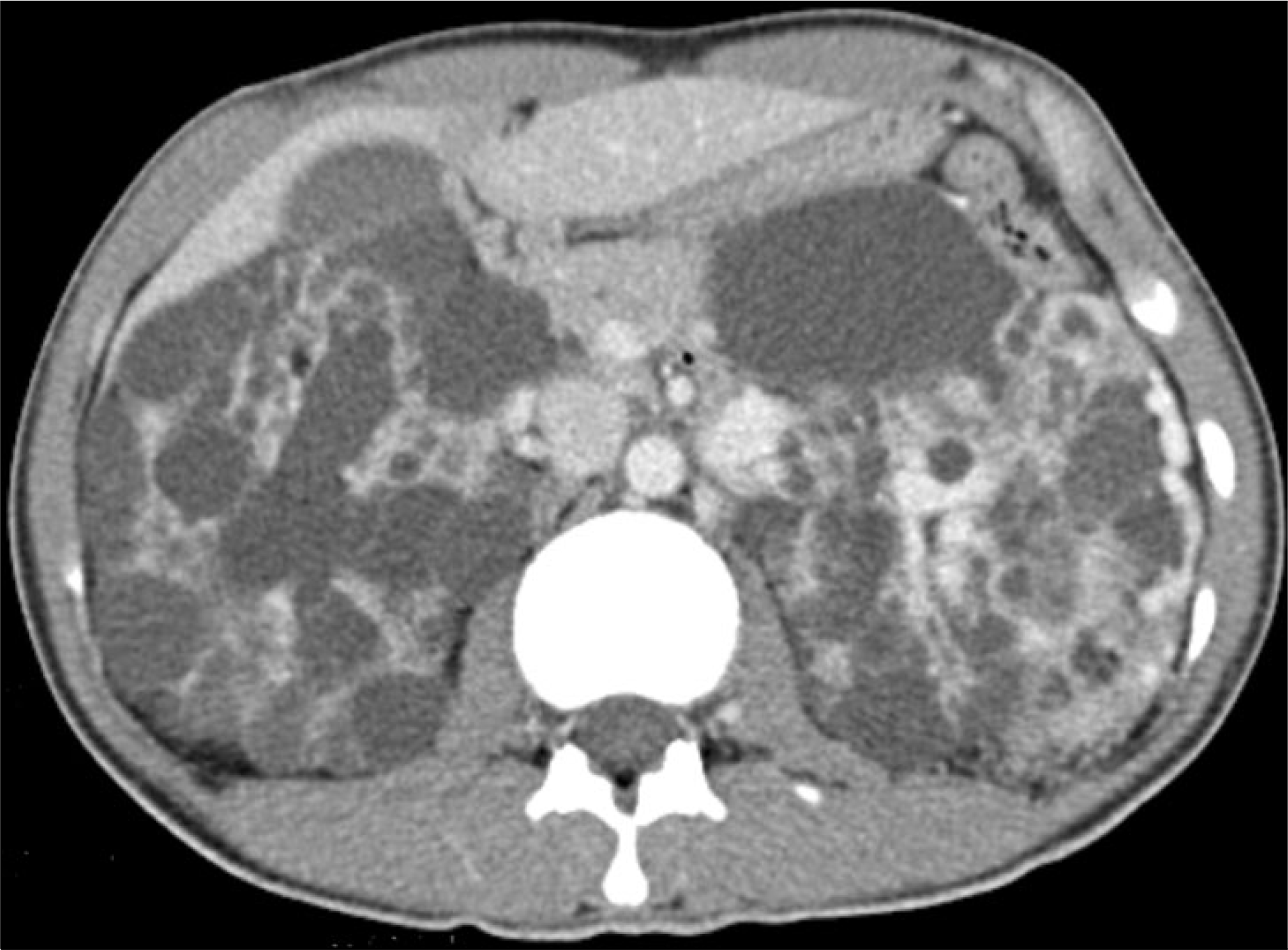
Fig. 2.
Contrast enhanced abdominal computed tomography (CT) image. Abdominal CT scan shows huge, well-marginated masses composed of both fat and soft tissue density in both kidneys.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download