Abstract
Background
High model for end-stage liver disease (MELD) scores (≥35) is closely associated with poor posttransplantation out-comes in patients who undergo living donor liver transplantation (LDLT). There is little information regarding factors that negatively impact the survival of patients with high MELD scores. The aim of this study was to identify factors associated with 3-month mortality of patients after LDLT.
Methods
We retrospectively analyzed 774 patients who underwent adult LDLT with right lobe grafts between 1996 and 2012. Exclusion criteria were retransplantation, left graft, auxiliary partial orthotopic liver transplantation, and inadequate medical recording. Preoperative variables were analyzed retrospectively.
Results
The overall 3-month survival rate was 92%. In univariate analysis, acute progression of disease, severity of hepatic encephalopathy, Child-Pugh class C, hepatorenal syndrome, use of continuous renal replacement therapy, use of ventilator, intensive care unit (ICU) care before transplantation, and MELD scores ≥35 were identified as potential risk factors. However, only ICU care before transplantation and MELD scores ≥35 were independent risk factors for 3-month mortality after LDLT. Three-month and 1-year patient survival rates for those with no risk factors were 95.5% and 88.6%, respectively. In contrast, patients with at least one risk factor had 3-month and 1-year patient survival rates of 88.4% and 81.1%, respectively, while patients with two risk factors had 3-month and 1-year patient survival rates of 55.6% and 55.6%, respectively.
Go to : 
References
1). Korean Network for Organ Sharing (KONOS). Korean Network for Organ Sharing [Internet]. Seoul: KONOS;2014. [cited 2014 Jun 30]. Available from:. http://www.konos.go.kr/konosis/index.jsp.
2). Ikegami T, Shirabe K, Yoshiya S, Yoshizumi T, Ninomiya M, Uchiyama H, et al. Bacterial sepsis after living donor liver transplantation: the impact of early enteral nutrition. J Am Coll Surg. 2012; 214:288–95.

3). Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008; 8:2569–79.

4). Li C, Mi K, Wen T, Yan L, Li B, Yang J, et al. Outcomes of patients with benign liver diseases undergoing living donor versus deceased donor liver transplantation. PLoS One. 2011; 6:e27366.

5). Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: strengths and weaknesses. J Hepatol. 2011; 54:1297–306.

6). Trotter JF, Osgood MJ. MELD scores of liver transplant recipients according to size of waiting list: impact of organ allocation and patient outcomes. JAMA. 2004; 291:1871–4.
7). Onaca NN, Levy MF, Sanchez EQ, Chinnakotla S, Fasola CG, Thomas MJ, et al. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver Transpl. 2003; 9:117–23.

8). Selzner M, Kashfi A, Cattral MS, Selzner N, McGilvray ID, Greig PD, et al. Live donor liver transplantation in high MELD score recipients. Ann Surg. 2010; 251:153–7.

9). Li C, Wen T, Yan L, Li B, Wang W, Xu M, et al. Does model for end-stage liver disease score predict the short-term outcome of living donor liver transplantation? Transplant Proc. 2010; 42:3620–3.

10). Shin M, Song S, Kim JM, Kwon CH, Kim SJ, Lee SK, et al. Donor morbidity including biliary complications in living-donor liver transplantation: singlecenter analysis of 827 cases. Transplantation. 2012; 93:942–8.
11). Moon JI, Kwon CH, Joh JW, Jung GO, Choi GS, Park JB, et al. Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl. 2010; 16:864–9.

12). Kim JM, Kim SJ, Joh JW, Kwon CH, Song S, Shin M, et al. Is cytomegalovirus infection dangerous in cytomegalovirus-seropositive recipients after liver transplantation? Liver Transpl. 2011; 17:446–55.

13). Foxton MR, Al-Freah MA, Portal AJ, Sizer E, Bernal W, Auzinger G, et al. Increased model for end-stage liver disease score at the time of liver transplant results in prolonged hospitalization and overall intensive care unit costs. Liver Transpl. 2010; 16:668–77.

14). New York State Department of Health. Information for a Healthy New York [Internet]. New York: New York State Department of Health;2014. [cited 2014 Jul 3]. Available from:. http://www.health.ny.gov.
15). Hayashi PH, Trotter JF, Forman L, Kugelmas M, Steinberg T, Russ P, et al. Impact of pretransplant diagnosis of hepatocellular carcinoma on cadveric liver allocation in the era of MELD. Liver Transpl. 2004; 10:42–8.

16). Chok KS, Chan SC, Fung JY, Cheung TT, Chan AC, Fan ST, et al. Survival outcomes of right-lobe living donor liver transplantation for patients with high model for end-stage liver disease scores. Hepatobiliary Pancreat Dis Int. 2013; 12:256–62.

17). Ikegami T, Imai D, Wang H, Yoshizumi T, Yamashita Y, Ninomiya M, et al. D-MELD as a predictor of early graft mortality in adult-to-adult living-donor liver transplantation. Transplantation. 2014; 97:457–62.

18). Nadalin S, Schaffer R, Fruehauf N. Split-liver transplantation in the high-MELD adult patient: are we being too cautious? Transpl Int. 2009; 22:702–6.

19). Earl TM, Chari RS. Which types of graft to use in patients with acute liver failure? (A) Auxiliary liver transplant (B) Living donor liver transplantation (C) The whole liver. (C) I take the whole liver only. J Hepatol. 2007; 46:578–82.
20). Barr ML, Belghiti J, Villamil FG, Pomfret EA, Sutherland DS, Gruessner RW, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006; 81:1373–85.

21). Park I, Moon E, Hwang JA, Yu S, Kim BW, Wang HJ, et al. Does hepatorenal syndrome affect the result of liver transplantation? Clinical observations. Transplant Proc. 2010; 42:2563–6.

Go to : 
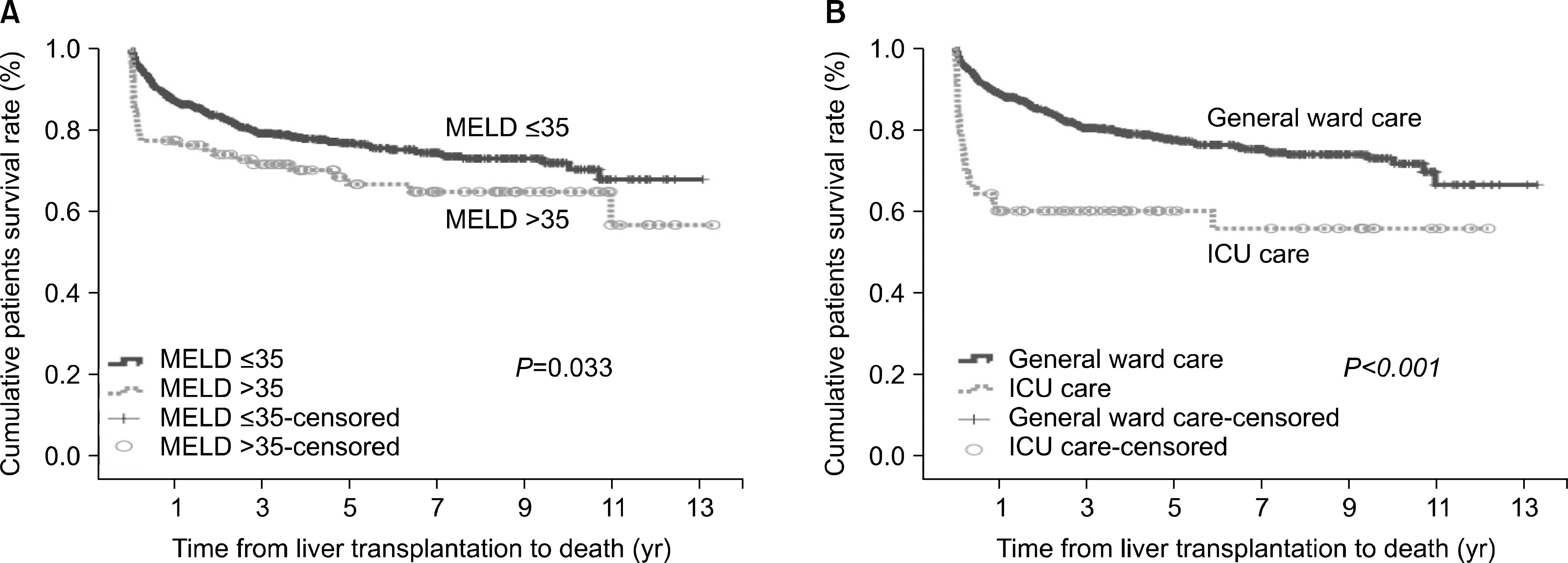 | Fig. 1.(A) End-stage liver disease (MELD) scores and (B) patients with pretransplant intensive care unit (ICU) care on patient survival. |
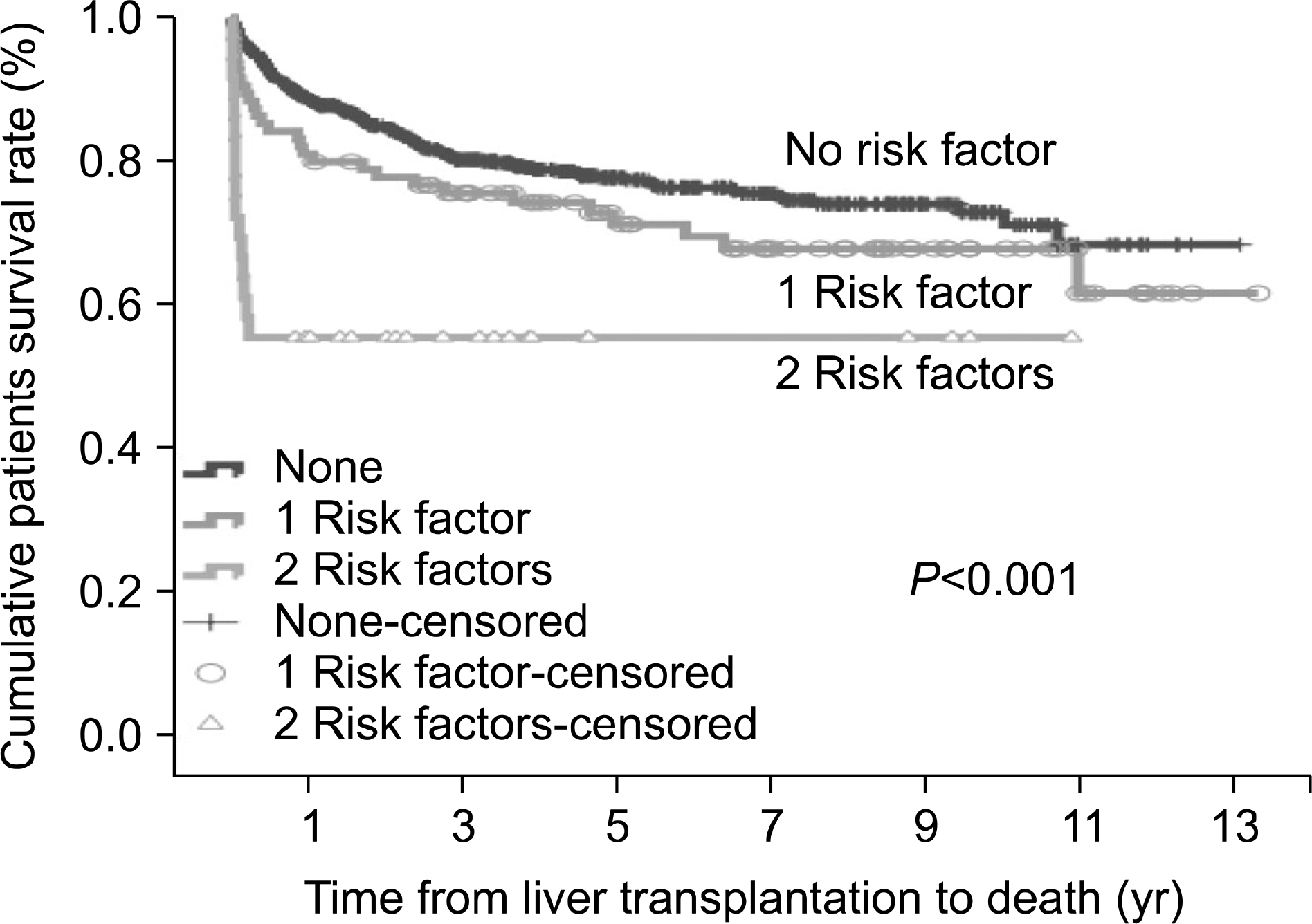 | Fig. 2.Patient survival with two risk factors which were high End-stage liver disease score (≥35) and pretransplant intensive care unit care when compared with those who had at least one risk factor or no risk factor. |
Table 1.
Causes of 3-month mortality after living donor liver transplantation
Table 2.
Comparison of patients with and without 3-month mortality after living donor liver transplantation
Table 3.
Risk factors for hospital mortality after living donor liver transplantation by multivariate analysis
| Variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Pretransplant ICU stay | 8.487 | 4.674∼15.408 | 0.000 |
| MELD ≥35 | 2.090 | 1.049∼4.164 | 0.036 |
Table 4.
Risk factor for 3-month mortality after living donor liver transplantation in patients requiring pretransplant intensive care
Table 5.
Risk factors for 3-month mortality after living donor liver transplantation in patients treated in the general ward pretransplant




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download