Abstract
Background
Immunosuppression after kidney transplantation is associated with increased risk of malignancy, which has become the second most common cause of death among kidney transplant recipients. In this review, we report the incidence of malignancies after kidney transplantation in a single center and evaluate the incidence, characteristics, relationship to immunosuppressive drugs and discuss what clinicians must consider during a follow-up of patients after kidney transplantation.
Methods
Between May 1978 and September 2013, a total of 748 kidney transplant patients who were able to undergo a follow-up process through electronic medical records were enrolled in this retrospective cohort study to determine the potential incidence and types of malignancy that may occur after kidney transplantation and the associated impact on patients and graft survival.
Results
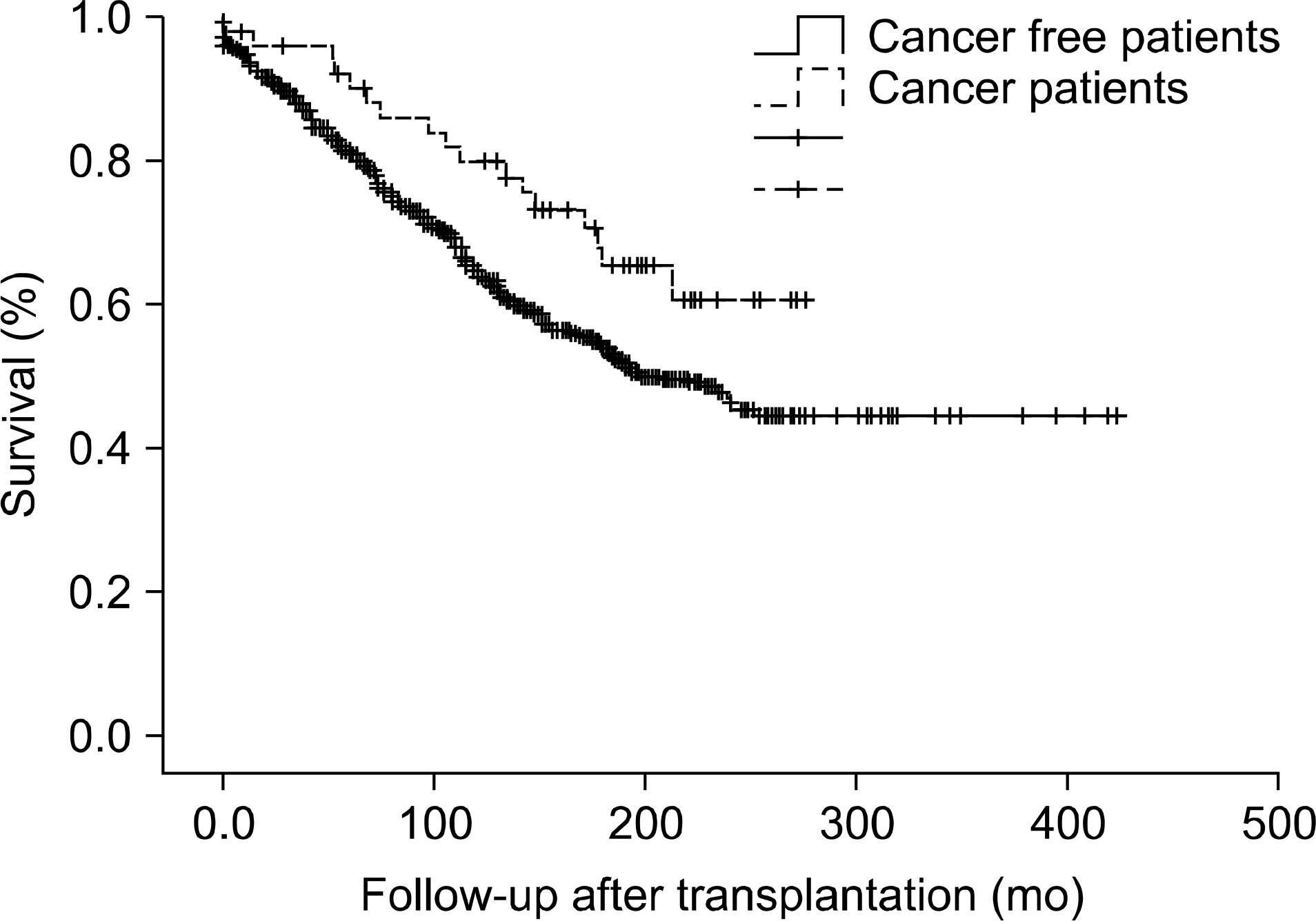
Among 748 patients, 63 cases of malignancy appeared in 54 patients (7.2%). Gastrointestinal cancer (12 cases, 19%) and post-transplant lymphoproliferative disorder (12 cases, 19%) were the two most common types of malignancy. The second most common type of malignancy was urinary tract malignancy in 10 patients. Two different types of malignancy were diagnosed in nine patients during our follow-up. The overall graft survival in malignancy patients was better, which may mean that malignancy did not affect the overall graft loss.
Go to : 
References
1). Marcé n R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009; 69:2227–43.
3). Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000; 342:605–12.

4). Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppression: de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007; 71:1271–8.
5). Vegso G, Tó th M, Hí dvé gi M, Toronyi E, Langer RM, Dinya E, et al. Malignancies after renal transplantation during 33 years at a single center. Pathol Oncol Res. 2007; 13:63–9.
7). Zeier M, Hartschuh W, Wiesel M, Lehnert T, Ritz E. Malignancy after renal transplantation. Am J Kidney Dis. 2002; 39:E5.

9). Fischereder M. Cancer in patients on dialysis and after renal transplantation. Nephrol Dial Transplant. 2008; 23:2457–60.

10). Swann PF, Waters TR, Moulton DC, Xu YZ, Zheng Q, Edwards M, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996; 273:1109–11.

11). Boratyń ska M, Watorek E, Falkiewicz K, Banasik M. Increased plasma TGF-beta 1 level in patient with salivary gland carcinoma after renal transplantation. Ann Transplant. 2005; 10:16–9.
12). Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anticancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004; 77:1777–82.

13). Dantal J, Pohanka E. Malignancies in renal transplantation: an unmet medical need. Nephrol Dial Transplant. 2007; 22(Suppl 1):i4–10.

14). Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004; 4:905–13.

15). Ro H, Kim SM, Kim KW, Hwang YH, Yang JS, Oh KH, et al. Malignancy after kidney transplantation. J Korean Soc Transplant. 2006; 20:187–92. (노한, 김선문, 김기원, 황영환, 양재석, 오국환, 등. 신이식 후 발생한 악성종양: 단일기관에서의 37년 발생양상 분석. 대한이식학회지 2006;20: 187–92.).
16). Kim HS, Seo YM, Park UJ, Kim HT, Cho WH, Hwang EA, et al. Crude incidence rate of malignancy after kidney transplantation. J Korean Soc Transplant. 2010; 24:182–6. (김효선, 서영민, 박의준, 김형태, 조원현, 황은아, 등. 면역억제제 노출기간을 고려한 신이식 후 악성종양의 조발생률. 대한이식학회지 2010;24: 182–6.).

18). Berber I, Altaca G, Aydin C, Dural A, Kara VM, Yigit B, et al. Kaposi's sarcoma in renal transplant patients: predisposing factors and prognosis. Transplant Proc. 2005; 37:967–8.

19). Sheil AG, Disney AP, Mathew TH, Amiss N. De novo malignancy emerges as a major cause of morbidity and late failure in renal transplantation. Transplant Proc. 1993; 25(1 Pt 2):1383–4.
20). Smith JM, Rudser K, Gillen D, Kestenbaum B, Seliger S, Weiss N, et al. Risk of lymphoma after renal transplantation varies with time: an analysis of the United States Renal Data System. Transplantation. 2006; 81:175–80.
21). Utada M, Ohno Y, Hori M, Soda M. Incidence of multiple primary cancers and interval between first and second primary cancers. Cancer Sci. 2014; 105:890–6.

22). Tremblay F, Fernandes M, Habbab F, de BEMD, Loertscher R, Meterissian S. Malignancy after renal transplantation: incidence and role of type of immunosuppression. Ann Surg Oncol. 2002; 9:785–8.

23). Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004; 4:87–93.

24). Bang BK. Malignancy in renal transplant recipients. Korean J Nephrol. 2006; 25:S497–503.
Go to : 
Table 1.
Characteristics of patients
Table 2.
Type of malignancy and time of diagnosis after renal transplantation
| Variable | No. (%) | Mean age at diagnosis (yr) | Mean time of diagnosis (mo) a | Response to treatment | |
|---|---|---|---|---|---|
| Good | Poor | ||||
| Stomach cancer | 7 (11.1) | 53.7±8.8 (40∼68) | 127.9±42.6 (63∼178) | 5 | 2 |
| Colon cancer | 2 (3.2) | 43.5±6.4 (39∼48) | 128.5±91.2 (64∼193) | 1 | 1 |
| Hepatocellular carcinoma | 2 (3.2) | 50.0±11.3 (42∼58) | 98.5±115.2 (17∼180) | 0 | 2 |
| pancreas tumor | 1 (1.6) | 66 | 149 | 1 | 0 |
| Lymphoma | 12 (19) | 45.4±10.8 (24∼60) | 104.3±59.8 (12∼204) | 9 | 3 |
| Kaposi sarcoma | 4 (6.3) | 48.2±15.4 (31∼66) | 50.5±68.1 (4∼149) | 3 | 1 |
| Skin cancer | 2 (3.2) | 63.5±9.2 (57∼70) | 123.5±82.7 (65∼182) | 1 | 1 |
| Breast cancer | 4 (6.3) | 50.0±14.4 (30∼63) | 53.5±40.0 (18∼108) | 2 | 2 |
| Thyroid cancer | 6 (9.5) | 50.8±9.8 (37∼65) | 129.3±55.0 (68∼193) | 6 | 0 |
| Urinary tract cancer | 10 (15.9) | 56.8±8.4 (44∼68) | 104.6±75.3 (10∼251) | 10 | 0 |
| Prostate cancer | 2 (3.2) | 66.0±2.8 (64∼68) | 126.5±61.5 (83∼170) | 2 | 0 |
| Female genital cancer | 7 (11.1) | 46.4±9.4 (38∼62) | 109.6±71.9 (16∼224) | 6 | 1 |
| Lung cancer | 3 (4.8) | 57.0±9.2 (49∼67) | 28.3±14.5 (14∼43) | 0 | 3 |
| Brain tumor | 1 (1.6) | 59 | 65 | 1 | 0 |
| Total | 63 (100) | 51.7±10.9 (24∼70) | 101.7±63.7 (4∼251) | 47 | 16 |
Table 3.
Patients with multiple primary malignancies
| Sex/age | First malignancy | Second malignancy | ||||
|---|---|---|---|---|---|---|
| Type | Pathologic diagnosis (stage) | Time of diagnosis (mo) a | Type | Pathologic diagnosis (stage) | Time of diagnosis (mo) a | |
| M/56 | Stomach | Adenocarcinoma (I) | 147 | Urinary bladder | Urothelial carcinoma in situ | 147 |
| F/34 | Sigmoid colon | Adenocarcinoma (III) | 64 | Uterus | Endometrioid carcinoma (II) | 73 |
| M/54 | Pancreas | IPMN b | 149 | Skin, forearm | Kaposi's sarcoma | 150 |
| M/22 | PTLD | Diffuse large B cell lymphoma | 30 | Thyroid | Papillary carcinoma (I) | 189 |
| M/59 | PTLD | Diffuse large B cell lymphoma | 12 | Urinary bladder | Urothelial carcinoma in situ | 86 |
| M/55 | Skin, left 3rd toe | Kaposi's sarcoma | 4 | Skin, nose | Basal cell carcinoma | 182 |
| M/36 | Thyroid | Papillary carcinoma (I) | 112 | Kidney, native | Papillary renal cell carcinoma (I) | 167 |
| M/47 | Kidney, native | Renal cell carcinoma (I) | 26 | Lung | Papillary adenocarcinoma | 28 |
| M/52 | Lung | Bronchioloalveolar carcinoma (II) | 43 | Kidney, native | Papillary adenoma | 49 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download