Abstract
Diabetes mellitus is one of the leading metabolic diseases that cause an increasing rate of mortality and morbidity. Recently, rather than the current drug treatment, pancreatic islet transplantation has been regarded as a potentially promising strategy for insulindependent diabetes mellitus while preventing complications such as kidney damage, vascular damage, nerve damage, and blindness. Recently, a number of advanced islet encapsulation techniques have been designed to enhance the efficiency of islet transplantation, including cell sheet engineering and generation of 3D islet spheroids by high density suspension system (HDSS). Chondrocytes derived from cartilage sources have been used as an encapsulation biomaterial for islets not only for autograft but also for allograft and xenograft transplantation. Cartilage is an avascular, white connective tissue that is rich in extracellular matrix, and expandable in vitro. Hence, this tissue might have immunologically privileged properties that make it an intelligent cell source for manufacture of encapsulation biomaterials. However, cell sheet engineering and HDSS still have their respective limitations, which need to be elucidated. This review will describe the advantages and disadvantages of the current encapsulation techniques in order to provide a comprehensive foundation for further modifications and improvements of tissue engineering for islet transplantation.
References
1). Sutherland DE. Pancreas and islet transplantation. II. Clinical trials. Diabetologia. 1981; 20:435–50.
2). Robertson RP. Islet transplantation as a treatment for diabetes− a work in progress. N Engl J Med. 2004; 350:694–705.
4). Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343:230–8.

5). Kaufman DB, Gores PF, Field MJ, Farney AC, Gruber SA, Stephanian E, et al. Effect of 15-deoxyspergualin on immediate function and longterm survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes. 1994; 43:778–83.

6). Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999; 48:1907–14.

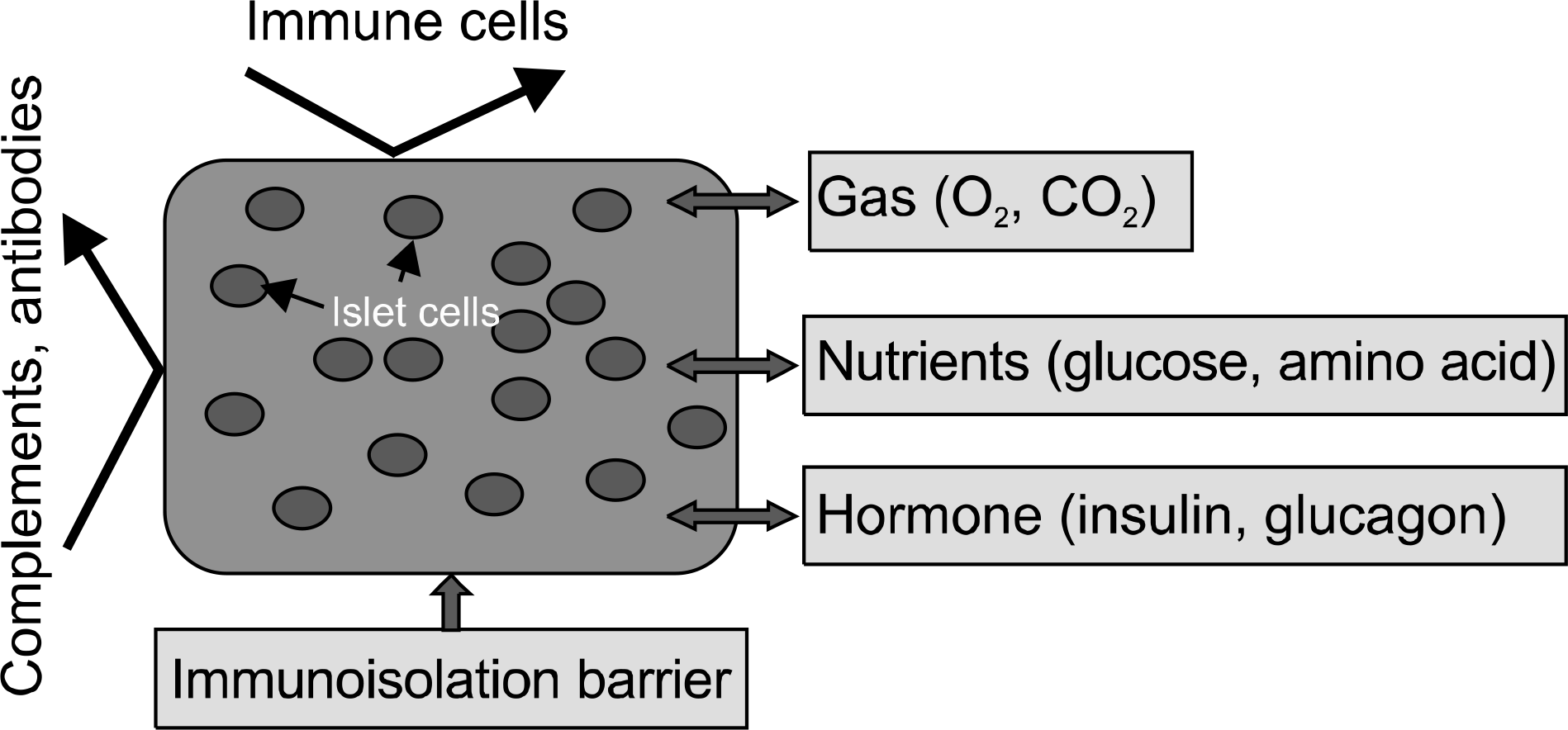
7). de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006; 27:5603–17.

8). Park YJ, Ahn HJ, Kim YS, Cho Y, Joo DJ, Ju MK. Illumina-mi-croarray analysis of mycophenolic acid-induced cell death in an insulin-producing cell line and primary rat islet cells: new insights into apoptotic pathways involved. Cell Signal. 2010; 22:1773–82.

9). Kim JY, Yoon SY, Park J, Kim YS. Mycophenolic acid induces islet apoptosis by regulating mitogen-activated protein kinase activation. Transplant Proc. 2006; 38:3277–9.

10). Kim JY, Huh KH, Park YJ, Fang Y, Kang CM, Kim YS. Molecular mechanisms of cell death of mycophenolic acid-treated primary isolated rat islets: implication of mi-togen-activated protein kinase activation. Transplant Proc. 2008; 40:2575–7.

11). Lanza RP, Sullivan SJ, Chick WL. Perspectives in diabetes. Islet transplantation with immunoisolation. Diabetes. 1992; 41:1503–10.

12). Wang T, Lací k I, Brissová M, Anilkumar AV, Prokop A, Hunkeler D, et al. An encapsulation system for the immunoisolation of pancreatic islets. Nature Biotechnology. 1997; 15:358–62.

13). Mikos AG, Papadaki MG, Kouvroukoglou S, Ishaug SL, Thomson RC. Mini-review: Islet transplantation to create a bioartificial pancreas. Biotechnol Bioeng. 1994; 43:673–7.

14). de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med. 2002; 8:363–6.

15). Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet encapsulation: strategies to enhance islet cell functions. Tissue Eng. 2007; 13:589–99.

16). Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozoto-cin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010; 90:1054–62.

17). Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000; 42:29–64.

18). de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002; 45:159–73.

19). Orive G, Herná ndez RM, Gascó n AR, Calafiore R, Chang TM, De Vos P, et al. Cell encapsulation: promise and progress. Nat Med. 2003; 9:104–7.

20). Zielinski BA, Aebischer P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 1994; 15:1049–56.

21). Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980; 210:908–10.

22). Hunt NC, Grover LM. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010; 32:733–42.

23). Iwata H, Amemiya H, Matsuda T, Takano H, Hayashi R, Akutsu T. Evaluation of microencapsulated islets in agarose gel as bioartificial pancreas by studies of hormone secretion in culture and by xenotransplantation. Diabetes. 1989; 38(Suppl 1):224–5.

24). Dawson RM, Broughton RL, Stevenson WT, Sefton MV. Microencapsulation of CHO cells in a hydroxyethyl meth-acrylate-methyl methacrylate copolymer. Biomaterials. 1987; 8:360–6.

25). Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, et al. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly (ethylene glycol) diacrylate membranes. Cell Transplant. 1999; 8:293–306.
26). Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomater. 2006; 2:1–8.
27). De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation. 1997; 63:824–30.
28). Teramura Y, Iwata H. Islet encapsulation with living cells for improvement of biocompatibility. Biomaterials. 2009; 30:2270–5.

29). Lee JI, Nishimura R, Sakai H, Sasaki N, Kenmochi T. A newly developed immunoisolated bioartificial pancreas with cell sheet engineering. Cell Transplant. 2008; 17:51–9.

30). Pollok JM, Lorenzen M, Kö lln PA, Tö rö k E, Kaufmann PM, Kluth D, et al. In vitro function of islets of Langerhans encapsulated with a membrane of porcine chondrocytes for immunoisolation. Dig Surg. 2001; 18:204–10.

31). Beenken-Rothkopf LN, Karfeld-Sulzer LS, Davis NE, Forster R, Barron AE, Fontaine MJ. The incorporation of extracellular matrix proteins in protein polymer hydrogels to improve encapsulated beta-cell function. Ann Clin Lab Sci. 2013; 43:111–21.
32). Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, et al. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012; 33:6691–7.

33). Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994; 331:889–95.

34). Basad E, Ishaque B, Bachmann G, Stü rz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010; 18:519–27.

35). Mikuriya T, Sugahara K, Shimogori H, Miura M, Yamashita H. Evaluation of deformation after auricular cartilage collection. Nihon Jibiinkoka Gakkai kaiho. 2002; 105:887–92.

36). Van Osch GJ, Mandl EW, Jahr H, Koevoet W, Nolst-Trenité G, Verhaar JA. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004; 41:411–21.
37). Sun L, Reagan MR, Kaplan DL. Role of cartilage-forming cells in regenerative medicine for cartilage repair. Orthop Res Rev. 2010; 2010:85–94.
38). El Sayed K, Haisch A, John T, Marzahn U, Lohan A, Mü ller RD, et al. Heterotopic autologous chondrocyte transplantation− a realistic approach to support articular cartilage repair? Tissue Eng Part B Rev. 2010; 16:603–16.
39). Yanaga H, Imai K, Koga M, Yanaga K. Cell-engineered human elastic chondrocytes regenerate natural scaffold in vitro and neocartilage with neoperichondrium in the human body posttransplantation. Tissue Eng Part A. 2012; 18:2020–9.

40). Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional twoand three-di-mensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007; 13:880–5.
41). Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y, et al. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol Chem Rapid Commun. 1990; 11:571–6.
42). Kim JY, Lee JI, Jeong JH, Park YJ, Kim SJ, Fang YH, et al. Functional evaluation of chondrocyte sheeting immuno-delusive immunoisolated bioartificial pancreas. Transplant Proc. 2010; 42:903–6.

43). Kim JY, Kim HW, Bae SJ, Joo DJ, Huh KH, Fang YH, et al. Hybrid cellular spheroids from hepatocellular carcinoma and insulin-secreting cell lines. Transplant Proc. 2012; 44:1095–8.

44). Lee JI, Kim JY, Kim HW, Bae SJ, Joo DJ, Huh KH, et al. Long-term viability of transplanted hybrid cellular spheroids within chondrocyte sheets. Transplant Proc. 2012; 44:1162–5.

45). Vé riter S, Gianello P, Dufrane D. Bioengineered sites for islet cell transplantation. Current diabetes reports. 2013; 13:745–55.
Fig. 2.
Morphology of chondrocyte sheeting immunoisolated-bioartificial pancreas (CSI-BAP). (A) The gross observation of CSI-BAP, (B) phase contrast at day 5, and (C, D) hematoxylin and eosin stain and azan staining (×40).
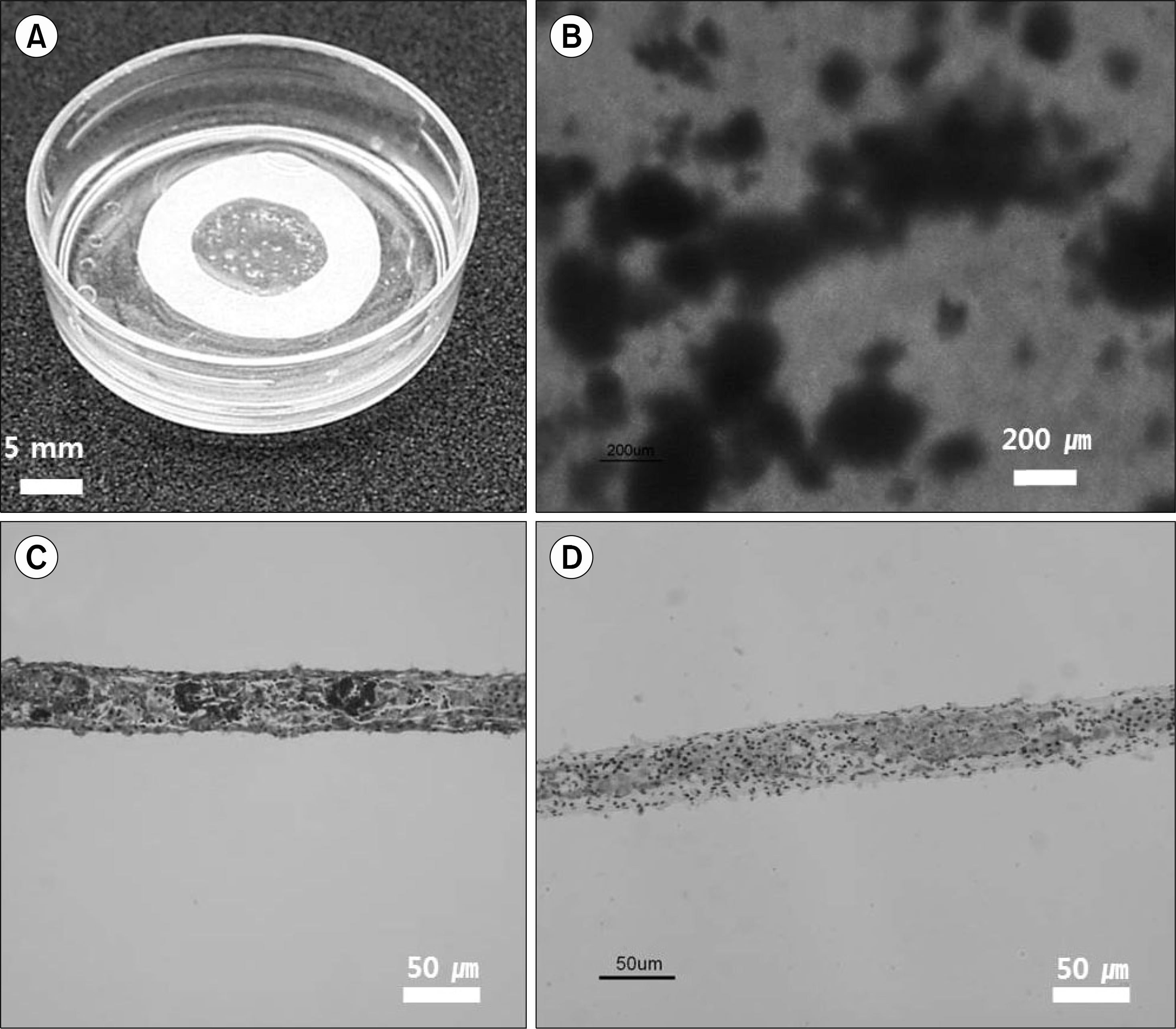
Fig. 3.
Morphology of chondrocytes microencapsulating immunoisolated (CMI) islet. (A, B) Macroscopic observation, structure of CMI-islet were in the rage of approximately 200∼600 m, (C) histological evaluation with azan stain, and (D) immunohistochemistry for insulin confirmed insulin secretion within the cells of pancreatic islet showing islets of CMI-islets.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download