Abstract
The recipient candidate was a 51-year-old male with end-stage renal disease owing to diabetes mellitus. The initial immunosuppressive regimen included basiliximab for induction and tacrolimus, mycophenolate mofetil, and steroids. Urine output was 413 mL/day on the operative day and 100 mL/day on the postoperative day (POD) 1. There was no definite stenosis of the ureter or vessels. He had anuria on POD 2∼4 and he had undergone hemodialysis. His serum creatinine level did not decrease. Therefore, a graft biopsy was performed on POD 4. The pathologic finding was consistent with acute calcineurin inhibitor (CNI) toxicity. There was no evidence of rejection or acute tubular necrosis. Anuria continued on POD 6; therefore, we started sirolimus instead of a CNI based regimen. Graft function was gradually recovered 1 day after reduction of CNI dose and hemodialysis was stopped. The serum creatinine level was normalized on POD 10. He was discharged on POD 21.
Go to : 
References
1). Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978; 2:1323–7.

2). Calne RY, Rolles K, White DJ, Thiru S, Evans DB, McMaster P, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979; 2:1033–6.

3). Morales JM, Wramner L, Kreis H, Durand D, Campistol JM, Andres A, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002; 2:436–42.

4). Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant. 2004; 4:1776–85.

5). Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009; 4:481–508.

6). Shaffer D, Langone A, Nylander WA, Goral S, Kizilisik AT, Helderman JH. A pilot protocol of a calcineurin-inhibitor free regimen for kidney transplant recipients of marginal donor kidneys or with delayed graft function. Clin Transplant. 2003; 17(Suppl 9):31–4.

7). Pascual M, Curtis J, Delmonico FL, Farrell ML, Williams WW Jr, Kalil R, et al. A prospective, randomized clinical trial of cyclosporine reduction in stable patients greater than 12 months after renal transplantation. Transplantation. 2003; 75:1501–5.

8). Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized longterm trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation. II. Survival, function, and protocol compliance at 1 year. Transplantation. 2004; 77:252–8.

9). Groth CG, Bä ckman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999; 67:1036–42.
10). Johnson RW, Kreis H, Oberbauer R, Brattströ m C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001; 72:777–86.

11). Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP. Sirolimus Renal Function Study Group. Improved renal function in sirolimus-treated renal transplant patients after early cyclosporine elimination. Transplantation. 2002; 74:1560–7.
12). Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009; 87:233–42.

Go to : 
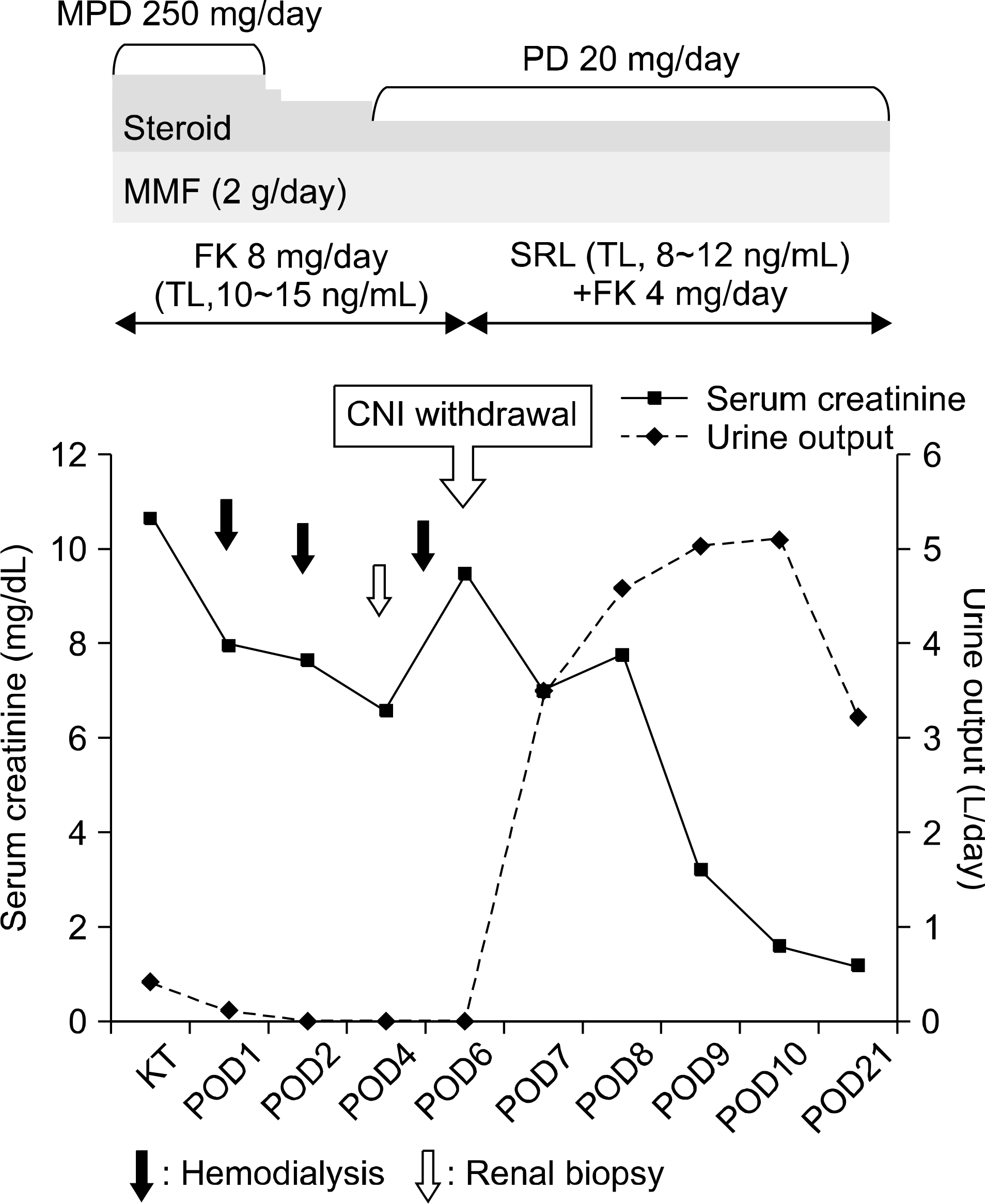 | Fig. 1.Course of urine output and serum creainine levels according to treatment. Abbreviations: KT, kidney transplantation; POD, postoperative day; CNI, calcineurin inhibitor; MPD, methylpred-nisolone; PD, prednisolone; MMF, mycophenolate mofetil; FK, tacrolimus; TL, trough level; SRL, sirolimus. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download