Abstract
Background
Kidney injury molecule-1 (KIM-1) is known as a good ancillary marker of acute kidney injury (AKI) and its expression has also been observed in acute rejection and chronic graft dysfunction. We tested usefulness of KIM-1 as an indicator of acute and chronic renal graft injury by correlating KIM-1 expression with renal graft function and histology.
Methods
A total of 133 zero-time biopsies and 42 follow-up biopsies obtained within 1 year posttransplantation were selected. Renal tubular KIM-1 staining was graded semiquantitatively from 0 to 3 and the extent of staining was expressed as the ratio of KIM-1 positive/CD10 positive proximal tubules using Image J program.
Results
KIM-1 was positive in 39.8% of zero-time biopsies. KIM-1 positive cases were predominantly male and had received grafts from donors with older age, deceased donors, and poor renal function at the time of donation, compared with KIM-1 negative cases. KIM-1 expression showed correlation with delayed graft function and acute tubular necrosis. In comparison of KIM-1 expression between stable grafts (n=23) and grafts with dysfunction (n=19) at the time of repeated biopsy, the intensity/extent of KIM-1 staining and renal histology at zero-time did not differ significantly between the two groups. Histologically, KIM-1 expression was significantly increased with both acute and chronic changes of glomeruli, tubules and interstitium, peritubular capil-laritis, and arteriolar hyalinosis.
Go to : 
References
1). Schrö ppel B, Krü ger B, Walsh L, Yeung M, Harris S, Garrison K, et al. Tubular expression of KIM-1 does not predict delayed function after transplantation. J Am Soc Nephrol. 2010; 21:536–42.
2). Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009; 4:873–82.

3). Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002; 62:237–44.

4). Nijboer WN, Schuurs TA, Damman J, van Goor H, Vaidya VS, van der Heide JJ, et al. Kidney injury molecule-1 is an early noninvasive indicator for donor brain death-induced injury prior to kidney transplantation. Am J Transplant. 2009; 9:1752–9.

5). Hong ME, Hong JC, Stepkowski S, Kahan BD. Correlation between cyclosporine-induced nephrotoxicity in reduced nephron mass and expression of kidney injury molecule-1 and aquaporin-2 gene. Transplant Proc. 2005; 37:4254–8.

6). Rouse RL, Zhang J, Stewart SR, Rosenzweig BA, Espandiari P, Sadrieh NK. Comparative profile of commercially Available urinary biomarkers in preclinical drug-induced kidney injury and recovery in rats. Kidney Int. 2011; 79:1186–97.

7). Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, et al. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007; 72:985–93.

8). Vinken P, Starckx S, Barale-Thomas E, Looszova A, Sonee M, Goeminne N, et al. Tissue Kim-1 and urinary clusterin as early indicators of cisplatin-induced acute kidney injury in rats. Toxicol Pathol. 2012; 40:1049–62.

9). Hoffmann D, Adler M, Vaidya VS, Rached E, Mulrane L, Gallagher WM, et al. Performance of novel kidney biomarkers in preclinical toxicity studies. Toxicol Sci. 2010; 116:8–22.

10). Szeto CC, Kwan BC, Lai KB, Lai FM, Chow KM, Wang G, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol. 2010; 5:2329–37.

11). van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation. 2007; 84:1625–30.

12). Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 2008; 73:608–14.

13). Kramer AB, van Timmeren MM, Schuurs TA, Vaidya VS, Bonventre JV, van Goor H, et al. Reduction of proteinuria in adriamycin-induced nephropathy is associated with reduction of renal kidney injury molecule (Kim-1) over time. Am J Physiol Renal Physiol. 2009; 296:F1136–45.

14). van Timmeren MM, Bakker SJ, Vaidya VS, Bailly V, Schuurs TA, Damman J, et al. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol. 2006; 291:F456–64.

15). Xu PC, Zhang JJ, Chen M, Lv JC, Liu G, Zou WZ, et al. Urinary kidney injury molecule-1 in patients with IgA nephropathy is closely associated with disease severity. Nephrol Dial Transplant. 2011; 26:3229–36.

16). Peters HP, Waanders F, Meijer E, van den Brand J, Steenbergen EJ, van Goor H, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant. 2011; 26:3581–8.

17). Nepal M, Bock GH, Sehic AM, Schultz MF, Zhang PL. Kidney injury molecule-1 expression identifies proximal tubular injury in urate nephropathy. Ann Clin Lab Sci. 2008; 38:210–4.
18). Nozaki Y, Kinoshita K, Yano T, Shiga T, Hino S, Niki K, et al. Estimation of kidney injury molecule-1 (Kim-1) in patients with lupus nephritis. Lupus. 2014; 23:769–77.

19). Kwon SH, Park MY, Jeon JS, Noh H, Choi SJ, Kim JK, et al. KIM-1 expression predicts renal outcomes in IgA nephropathy. Clin Exp Nephrol. 2013; 17:359–64.

20). van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury mole-cule-1 (KIM-1) in human renal disease. J Pathol. 2007; 212:209–17.

21). Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–60.

22). Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006; 290:F517–29.

23). Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Nephron Clin Pract. 2008; 109:c192–7.

24). Ting YT, Coates PT, Walker RJ, McLellan AD. Urinary tubular biomarkers as potential early predictors of renal allograft rejection. Nephrology (Carlton). 2012; 17:11–6.

25). Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010; 28:478–85.

Go to : 
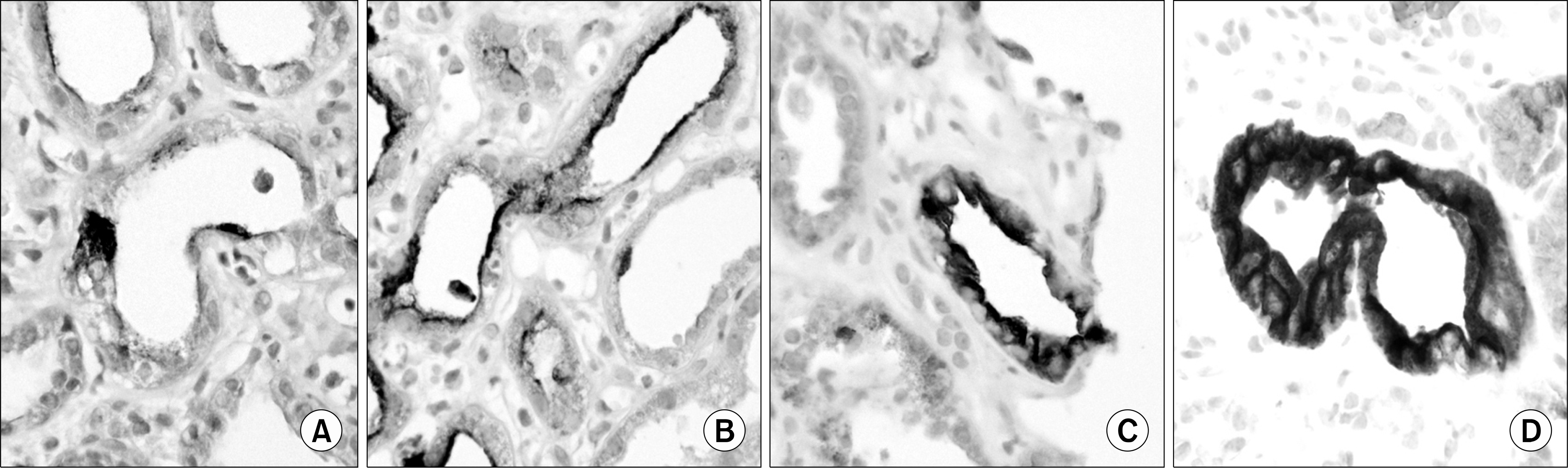 | Fig. 1.Scoring of renal tubular kidney injury molecule-1 expression: (A) 0.5, focal staining, (B) 1, weak and entire staining, (C) 2, moderate and entire staining. and (D) 3, strong and entire staining (×400). |
 | Fig. 2.Renal tubular kidney injury molecule-1 (KIM-1) expression in patients with stable graft function and dysfunction (×200). (A, B) In a patient with stable renal function, tubular KIM-1 expression increased in the repeat biopsy (B, score 2) compared with zero time (A, score 0.5). (C, D) Tubular KIM-1 expression became prominent in the repeat biopsy (D, score 3) in a patient with graft dysfunction, who showed negative KIM-1 staining at zero time (C). |
Table 1.
Patient’ s demography according to zero-time KIM-1 expression
Table 2.
Patient’ s demography according to zero-time KIM-1 staining intensity
Table 3.
Correlation between KIM-1 expression and light microscopic features
Table 4.
Changes of renal function and histology in KIM-1 positive cases at zero-time (n=5)
Table 5.
Changes of renal function and histology in KIM-1 negative cases at zero-time (n=37)
Table 6.
KIM-1 positivity and renal histology according to graft dysfunction at repeat biopsy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download