Abstract
Antibody-mediated rejection (AMR) by preformed and/or de novo human leukocyte antigen alloantibodies is a leading cause of early and late allograft loss. In this review, we describe strategic approaches to various forms of AMR in clinical settings that are not based on pathologic classification, which is controversial for atypical AMR (C4d-, DSA-, subclinical etc.). For acute AMR, a variety of modalities like plasmapheresis, intravenous immunoglobulin, and anti-CD20 antibodies have been utilized singly, or in combination, with variable results; however, no established treatment for chronic AMR is known. Significant research efforts are being made for developing new and novel therapies. Improvements in clinical outcomes can be expected from studies evaluating innovative therapeutic concepts, such as proteasome inhibition or complement-blocking agents.
REFERENCES
1). Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into crossmatch-positive recipients. Transplantation. 2000; 70:887–95.

2). Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359:242–51.

3). Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, et al. Terminal complement inhibition de-creases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011; 11:2405–13.

4). Crespo M, Pascual M, Tolkoff-Rubin N, Mauiyyedi S, Collins AB, Fitzpatrick D, et al. Acute humoral rejection in renal allograft recipients: I. incidence, serology and clinical characteristics. Transplantation. 2001; 71:652–8.
5). Lucas JG, Co JP, Nwaogwugwu UT, Dosani I, Suresh-kumar KK. Antibody-mediated rejection in kidney transplantation: an update. Expert Opin Pharmacother. 2011; 12:579–92.

6). Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria: an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003; 3:708–14.
7). Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, et al. A new diagnostic algorithm for anti-body-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012; 12:1168–79.

8). Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012; 12:563–70.

9). Haas M. Pathology of C4d-negative antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant [in press 2012 Dec 31].
10). Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guide-line for the care of kidney transplant recipients. Am J Transplant. 2009; 9(Suppl 3):S1–155.
11). Levine MH, Abt PL. Treatment options and strategies for antibody mediated rejection after renal transplantation. Semin Immunol. 2012; 24:136–42.

12). Bartel G, Schwaiger E, Bohmig GA. Prevention and treatment of alloantibody-mediated kidney transplant rejection. Transpl Int. 2011; 24:1142–55.

13). Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, et al. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003; 3:1017–23.

14). Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006; 1:421–32.

15). Montgomery RA, Cooper M, Kraus E, Rabb H, Sama-niego M, Simpkins CE, et al. Renal transplantation at the Johns Hopkins Comprehensive Transplant Center. Clin Transpl. 2003; 199–213.
16). Glotz D, Antoine C, Julia P, Pegaz-Fiornet B, Duboust A, Boudjeltia S, et al. Intravenous immunoglobulins and transplantation for patients with anti-HLA antibodies. Transpl Int. 2004; 17:1–8.

17). Schweitzer EJ, Wilson JS, Fernandez-Vina M, Fox M, Gutierrez M, Wiland A, et al. A high panel-reactive anti-body rescue protocol for crossmatch-positive live donor kidney transplants. Transplantation. 2000; 70:1531–6.
18). Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation. 2012; 94:775–83.

19). Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010; 10:464–71.

20). Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008; 8:2684–94.

21). Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, et al. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009; 9:1099–107.

22). Koller H, Steurer W, Mark W, Margreiter R, Lhotta K, Mayer G, et al. Clearance of C4d deposition after success-ful treatment of acute humoral rejection in follow-up bi-opsies: a report of three cases. Transpl Int. 2004; 17:177–81.

23). Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib provides effective therapy for antibody-and cell-mediated acute rejection. Transplantation. 2008; 86:1754–61.
24). Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009; 9:201–9.

25). Lonze BE, Dagher NN, Simpkins CE, Locke JE, Singer AL, Segev DL, et al. Eculizumab, bortezomib and kidney paired donation facilitate transplantation of a highly sensitized patient without vascular access. Am J Transplant. 2010; 10:2154–60.

26). Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement pro-tein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009; 9:231–5.

27). Locke JE, Zachary AA, Haas M, Melancon JK, Warren DS, Simpkins CE, et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant. 2007; 7:842–6.

28). Kaplan B, Gangemi A, Thielke J, Oberholzer J, Sankary H, Benedetti E. Successful rescue of refractory, severe antibody mediated rejection with splenectomy. Transplantation. 2007; 83:99–100.

29). Pascual J, Pérez-Sáez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando). 2012; 26:280–90.

30). de Kort H, Willicombe M, Brookes P, Dominy KM, Santos-Nunez E, Galliford JW, et al. Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transplant. 2013; 13:485–92.
31). Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011; 91:1103–9.

32). DeVos JM, Patel SJ, Burns KM, Dilioglou S, Gaber LW, Knight RJ, et al. De novo donor specific antibodies and patient outcomes in renal transplantation. Clin Transpl. 2011; 351–8.
33). Everly MJ. Donor-specific anti-HLA antibody monitor-ing and removal in solid organ transplant recipients. Clin Transpl. 2011; 319–25.
34). Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012; 12:388–99.

35). Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013; 95:410–7.

36). Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009; 88:568–74.

37). Willicombe M, Roufosse C, Brookes P, Galliford JW, McLean AG, Dorling A, et al. Antibody-mediated rejection after alemtuzumab induction: incidence, risk factors, and predictors of poor outcome. Transplantation. 2011; 92:176–82.

38). Ting A, Morris PJ. Powerful effect of HL-DR matching on survival of cadaveric renal allografts. Lancet. 1980; 2:282–5.

39). Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, et al. De novo DQ donor-specific antibodies are associated with a significant risk of anti-body-mediated rejection and transplant glomerulopathy. Transplantation. 2012; 94:172–7.

40). Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009; 9:2520–31.

41). Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013; 13:76–85.

42). Trivedi HL, Terasaki PI, Feroz A, Everly MJ, Vanikar AV, Shankar V, et al. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation. 2009; 87:1555–61.

43). Billing H, Rieger S, Ovens J, Susal C, Melk A, Waldherr R, et al. Successful treatment of chronic antibody-media-ted rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation. 2008; 86:1214–21.

44). Fehr T, Rusi B, Fischer A, Hopfer H, Wuthrich RP, Gaspert A. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation. 2009; 87:1837–41.

45). Flechner SM, Fatica R, Askar M, Stephany BR, Poggio E, Koo A, et al. The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation. 2010; 90:1486–92.

46). Walsh RC, Brailey P, Girnita A, Alloway RR, Shields AR, Wall GE, et al. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011; 91:1218–26.

47). Vogelbacher R, Meister S, Guckel E, Starke C, Wittmann S, Stief A, et al. Bortezomib and sirolimus inhibit the chronic active antibody-mediated rejection in experimental renal transplantation in the rat. Nephrol Dial Transplant. 2010; 25:3764–73.

48). Shah A, Nadasdy T, Arend L, Brennan J, Leong N, Coppage M, et al. Treatment of C4d-positive acute humoral rejection with plasmapheresis and rabbit polyclonal antithymocyte globulin. Transplantation. 2004; 77:1399–405.

49). Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005; 79:1507–15.

50). Colovai AI, Vasilescu ER, Foca-Rodi A, Kim-Schulze S, Hussaini N, D'Agati V D, et al. Acute and hyperacute humoral rejection in kidney allograft recipients treated with anti-human thymocyte antibodies. Hum Immunol. 2005; 66:501–12.

51). Tinckam KJ, Wood IG, Ji F, Milford EL. ATG induction is associated with an increase in anti-HLA antibodies after kidney transplantation. Hum Immunol. 2004; 65:1281–7.

52). Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002; 13:242–51.

53). Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci U S A. 2007; 104:14104–9.

54). Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009; 88:1–6.

55). Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-in-flammatory activity with a recombinant IgG Fc. Science. 2008; 320:373–6.

56). Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-in-flammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008; 105:19571–8.

57). Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006; 313:670–3.

58). Kazatchkine MD, Kaveri SV. Immunomodulation of au-toimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001; 345:747–55.

59). Casadei DH, del C Rial M, Opelz G, Golberg JC, Argento JA, Greco G, et al. A randomized and pro-spective study comparing treatment with high-dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid-resistant rejection. Transplantation. 2001; 71:53–8.

60). Jordan SC, Quartel AW, Czer LS, Admon D, Chen G, Fishbein MC, et al. Posttransplant therapy using high- dose human immunoglobulin (intravenous gammaglobu-lin) to control acute humoral rejection in renal and car-diac allograft recipients and potential mechanism of action. Transplantation. 1998; 66:800–5.
61). Luke PP, Scantlebury VP, Jordan ML, Vivas CA, Hakala TR, Jain A, et al. Reversal of steroid- and anti-lympho-cyte antibody-resistant rejection using intravenous immunoglobulin (IVIG) in renal transplant recipients. Transplantation. 2001; 72:419–22.
63). Gungor O, Sen S, Kircelli F, Yilmaz M, Sarsik B, Ozkahya M, et al. Plasmapheresis therapy in renal transplant patients: five-year experience. Transplant Proc. 2011; 43:853–7.

64). Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chi-meric mouse human monoclonal antibody to CD20. Blood. 1994; 83:435–45.

65). Ramanath V, Nistala R, Chaudhary K. Update on the role of rituximab in kidney diseases and transplant. Expert Opin Biol Ther. 2012; 12:223–33.

66). Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004; 4:996–1001.

67). Bellière J, Rostaing L, Guilbeau-Frugier C, Congy N, Kamar N. Low- versus high-dose rituximab for anti-body-mediated rejection after kidney transplantation. Transpl Int. 2013; 26:e12–4.

68). Brown CM, Abraham KA, O'Kelly P, Conlon PJ, Walshe JJ. Long-term experience of plasmapheresis in antibody- mediated rejection in renal transplantation. Transplant Proc. 2009; 41:3690–2.
69). Kaposztas Z, Podder H, Mauiyyedi S, Illoh O, Kerman R, Reyes M, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009; 23:63–73.

70). Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007; 7:402–7.

71). Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007; 84(12 Suppl):S33–6.

72). Danovitch GM. Handbook of kidney transplantation. 5th ed.Philadelphia: Lippincott Williams & Wilkins;2010.
73). Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003; 101:1530–4.

74). Walsh RC, Everly JJ, Brailey P, Rike AH, Arend LJ, Mogilishetty G, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010; 89:277–84.

75). Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006; 355:1233–43.

Fig. 1.
Target points of various therapeutic modalities. A sche-matic pathway of the steps needed to develop antibody-me-diated rejection (AMR) after transplantation and the discrete ac-tivities of the various therapeutic modalities against AMR are depicted. Reprinted from Fig. 1 of reference (11). Abbreviation: IVIG, intravenous immunoglobulin.
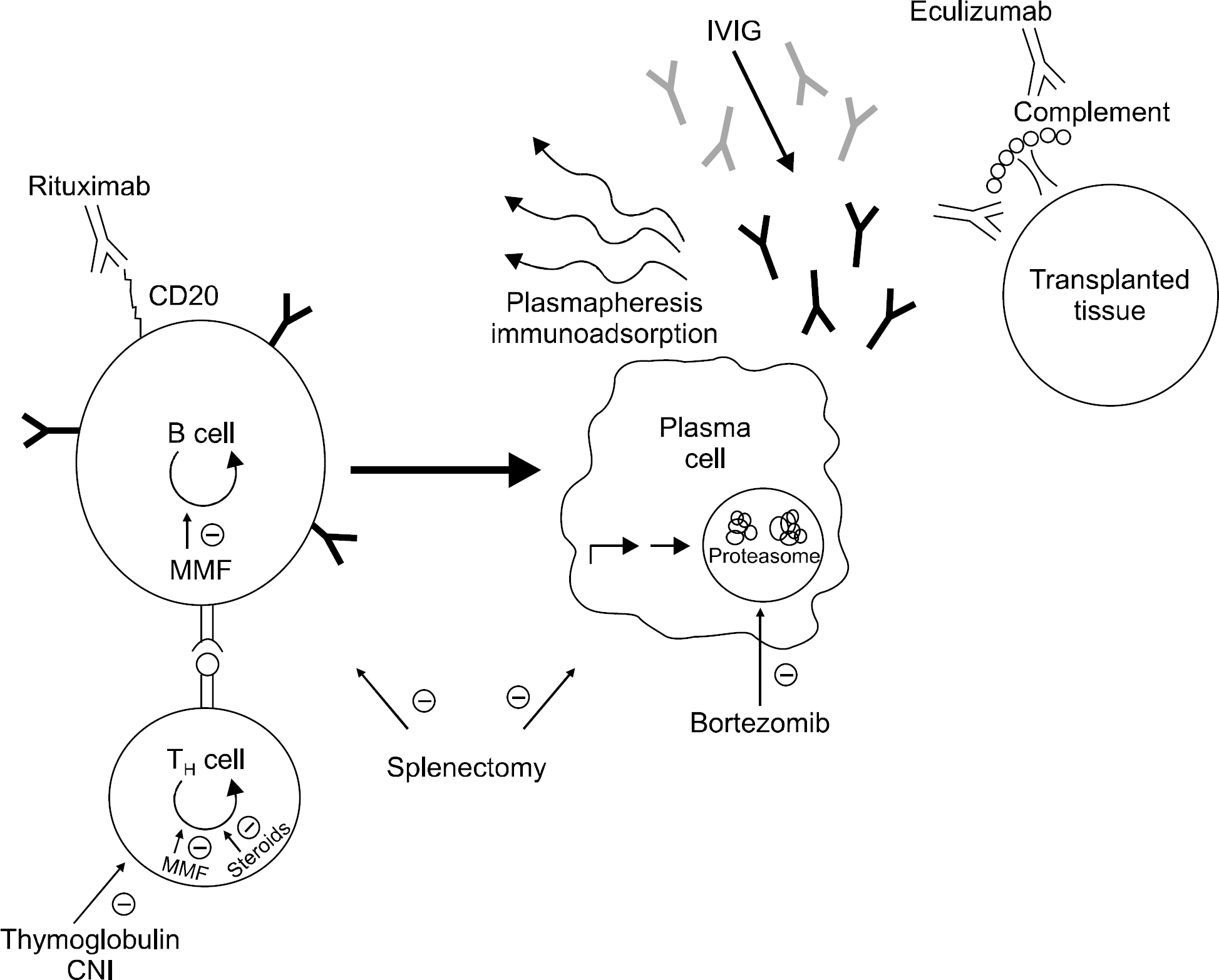
Fig. 2.
Antihumoral treatment concepts. Most protocols com-bine two major therapeutic principles: apheresis for antibody depletion and modulation of B cell immunity and other compo-nents of adaptive and innate immunity. Reprinted from Fig. 1 of reference (12). Abbreviations: IVIG, intravenous immunog-lobulin; ATG, antithymocyte globulin.
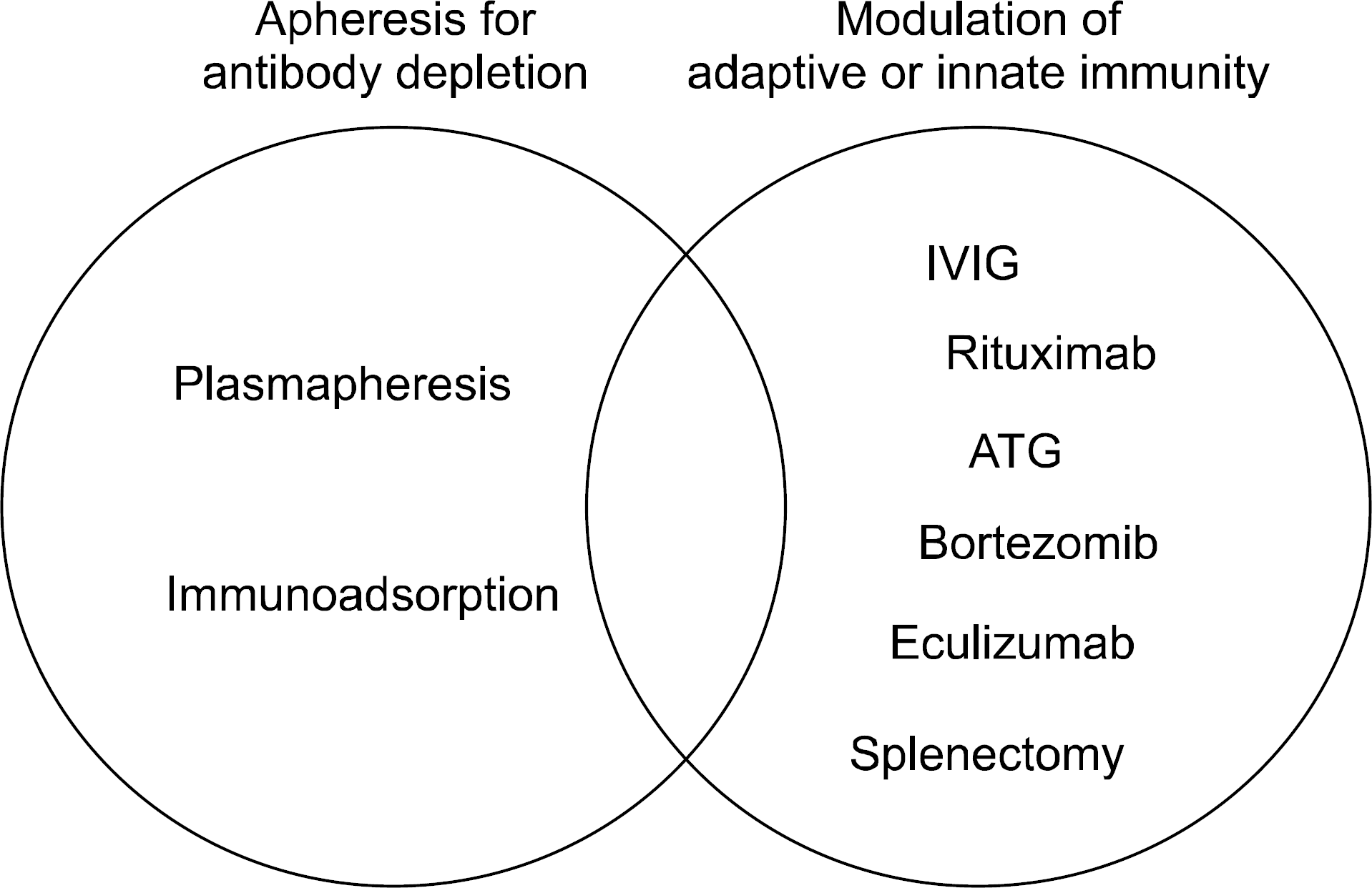
Fig. 3.
Therapeutic approach to acute antibody-mediated rejection (AMR). Reprinted from Fig. 3 of reference (5). Abbreviations: PRA, panel reactive antibody; IV, intravenous; DSA, donor specific antibody.
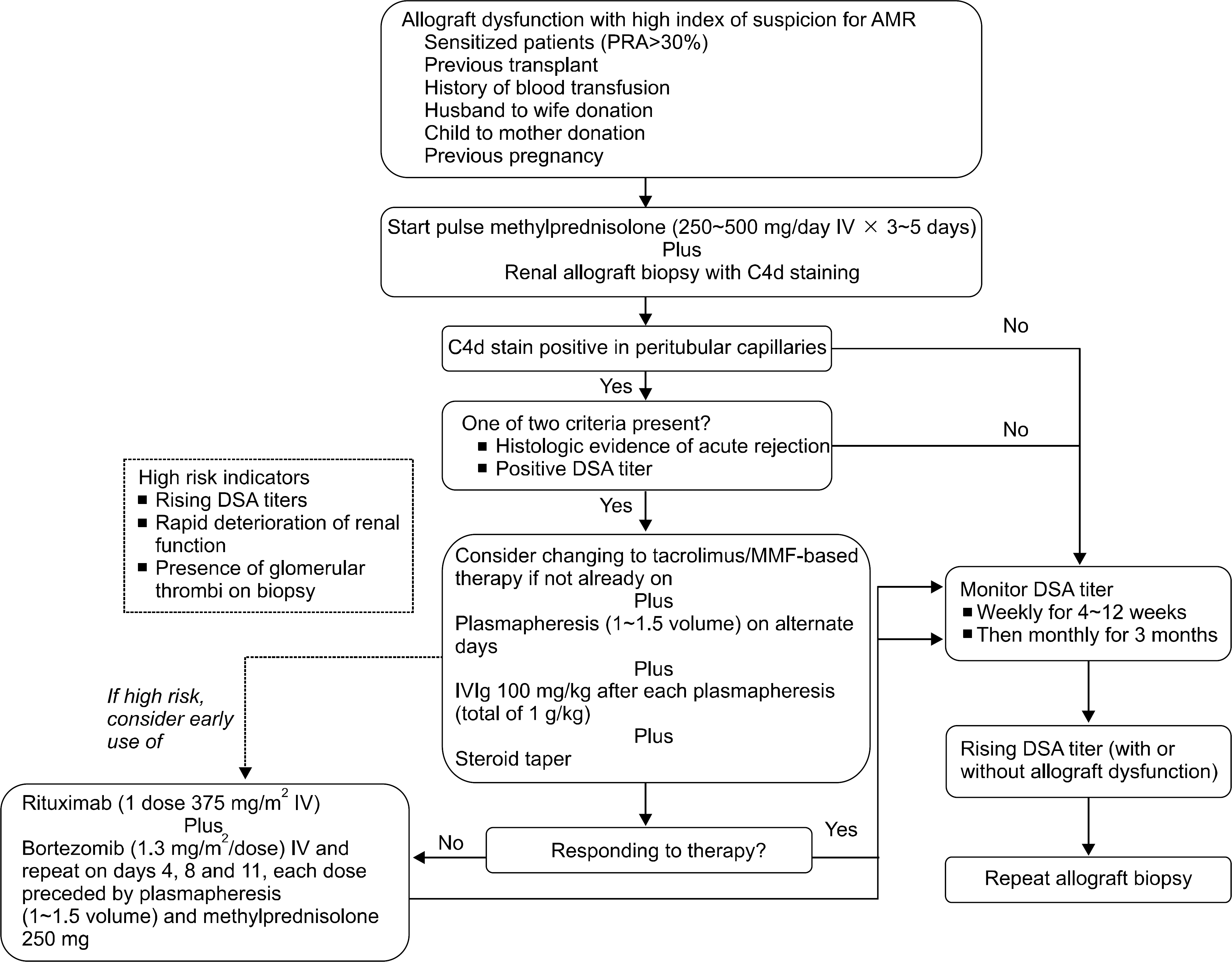
Table 1.
Summary of antibody-mediated changes in Banff '09 update a




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download