Abstract
Background:
Many in vitro experiments have demonstrated the immunosuppressive properties of mesenchymal stem cells (MSCs). However, such properties have not yet been fully established in an in vivo setting. The purpose of this study was to determine immunosuppressive and anti-inflammatory properties of MSCs in a preclinical animal model in order to pave the way for replacement of conventional immunosuppressive therapy.
Methods:
Male C57BL/6 mice and male BALB/c mice were chosen as skin graft donors and recipients, respectively. After performance of full-thickness skin transplantation on the back of mice, adipose tissue derived stem cells (1.0×106
/0.1 mL) stained with 4, 6-diamidino-2-phenylindole were transplanted into adipose tissue derived stem cell (ASC)-infused mice and phosphate buffered saline (PBS; 0.1 mL) was infused into PBS-infused mice. Immunological properties and graft survival were accessed and compared.
Results:
The serum levels of proinflammatory interleukin (IL)-6 showed a decrease in ASC-infused mice compared to PBS-infused mice (P<0.005). In addition, interferon-λ, IL-10, and tumor necrosis factor-α mRNA levels in the skin graft showed a decrease in ASC-infused mice, although without statistical significance. In ASC-infused mice, donor specific hyporesponsiveness was identified in a mixed lymphocyte reaction assay at 30 days after transplantation. In addition, ASC-infusion resulted in markedly prolonged skin allograft survival compared with PBS-infusion (P<0.001).
Conclusions:
Administration of ASC not only induced anti-inflammation and immunosuppression, but also resulted in prolonged graft survival, suggestive of their potent immunosuppressive properties. Therefore, conduct of further and more exquisite studies will be required in order to determine the role of MSC in the solid organ transplantation field in order to avoid adverse effects and toxicities caused by chemical immunosuppressive regimens.
Go to : 
REFERENCES
1). Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004; 13:117–30.

2). Anam K, Amare MF, Zins SR, Davis TA. Infusion of Lin- bone marrow cells results in multilineage macro-chimerism and skin allograft tolerance in minimally con-ditioned recipient mice. Transpl Immunol. 2010; 24:69–75.

3). Bartholomew A, Patil S, Mackay A, Nelson M, Buyaner D, Hardy W, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001; 12:1527–41.
4). Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002; 30:42–8.

5). Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838–43.

6). Hettiaratchy S, Melendy E, Randolph MA, Coburn RC, Neville DM Jr, Sachs DH, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004; 77:514–21.
7). Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008; 121:50–8.

8). Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxyge-nase-mediated tryptophan degradation. Blood. 2004; 103:4619–21.

9). Petit F, Minns AB, Nazzal JA, Hettiaratchy SP, Lantieri LA, Randolph MA, et al. Prolongation of skin allograft survival after neonatal injection of donor bone marrow and epidermal cells. Plast Reconstr Surg. 2004; 113:270–6.

10). Siemionow M, Demir Y, Mukherjee A, Klimczak A. Development and maintenance of donor-specific chimer-ism in semi-allogenic and fully major histocompatibility complex mismatched facial allograft transplants. Transplantation. 2005; 79:558–67.

11). Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–97.

12). Wang Y, Liu J, Xu C, Zhang W, Bai L, Li N, et al. Bone marrow transplantation combined with mesenchymal stem cells induces immune tolerance without cytotoxic con-ditioning. J Surg Res. 2011; 171:e123–31.

13). Zografou A, Tsigris C, Papadopoulos O, Kavantzas N, Patsouris E, Donta I, et al. Improvement of skin-graft survival after autologous transplantation of adipose-derived stem cells in rats. J Plast Reconstr Aesthet Surg. 2011; 64:1647–56.

14). Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008; 15:109–16.

15). Trento C, Dazzi F. Mesenchymal stem cells and innate tolerance: biology and clinical applications. Swiss Med Wkly. 2010; 140:w13121.

16). Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105:1815–22.

17). Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. 2012; 1:200–5.

18). Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006; 81:1589–95.

19). Kim SJ, Park KC, Lee JU, Kim KJ, Kim DG. Therapeu-tic potential of adipose tissue-derived stem cells for liver failure according to the transplantation routes. J Korean Surg Soc. 2011; 81:176–86.

20). Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF se-creted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011; 6:e17899.

21). Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, et al. Novel autologous cell therapy in is-chemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005; 25:2542–7.

22). Sbano P, Cuccia A, Mazzanti B, Urbani S, Giusti B, Lapini I, et al. Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch Dermatol Res. 2008; 300:115–24.

23). Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007; 262:509–25.

24). Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–506.

25). Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107:367–72.

26). Friedenstein AJ, Chailakhjan RK, Lalykina KS. The de-velopment of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970; 3:393–403.

27). Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and mono-cyte-derived dendritic cells. J Immunol. 2006; 177:2080–7.

Go to : 
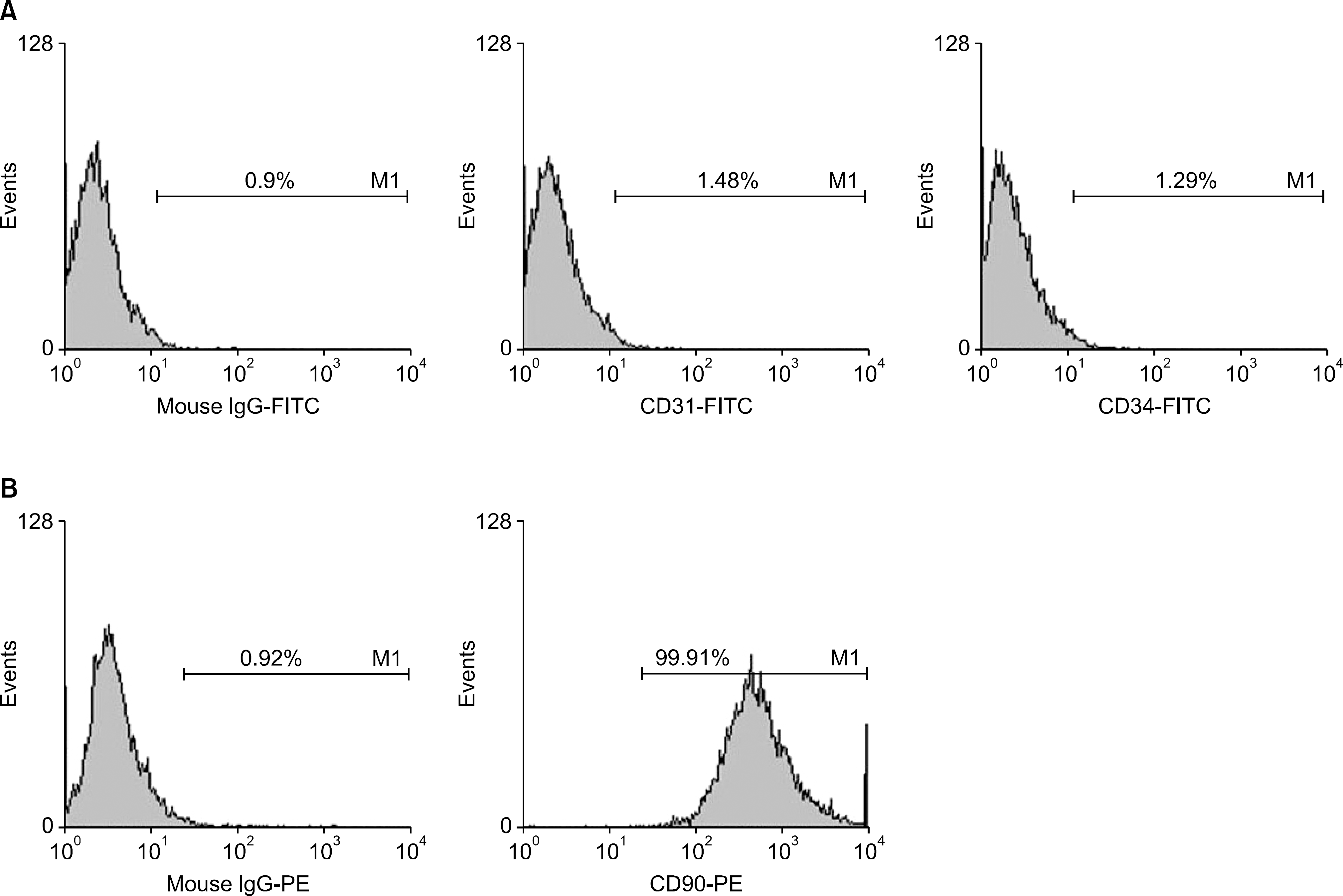 | Fig. 1.The expression of cell surface markers in adipose tissue-derived stem cells (ASCs). (A) Representative illustrations of flow cy-tometry demonstrating that ASC did not express hematopoietic stem cell marker (CD31, CD34), and (B) did express mesenchymal stem cell marker (CD90). |
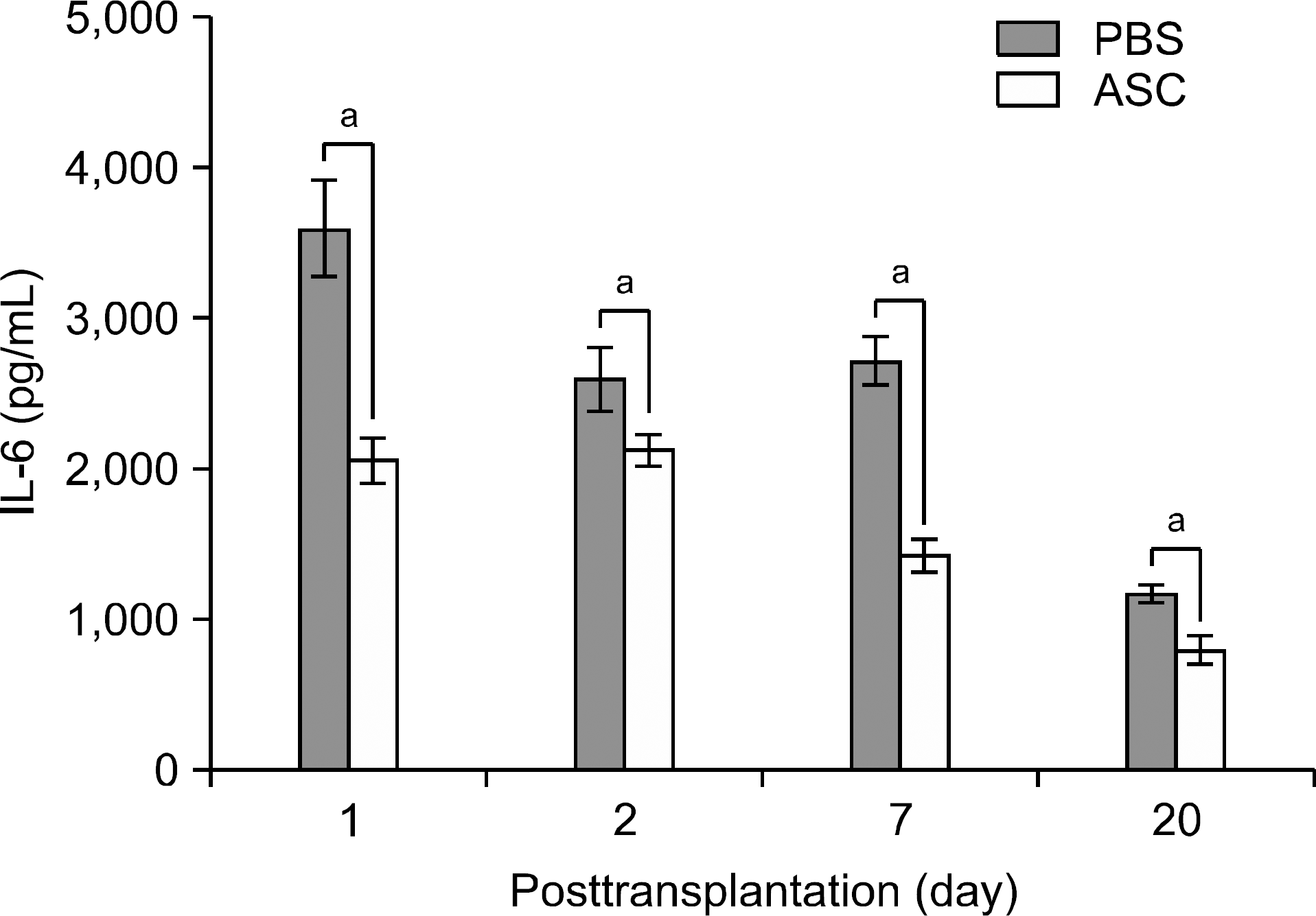 | Fig. 2.Comparison of the serum levels of proinflammatory cy-tokine interleukin (IL)-6 between phosphate buffered saline (PBS)-infused mice (n=16) and human adipose tissue derived stem cell (ASC)-infused mice (n=16) after skin allograft transfu-sion. Each of four mice in each group were measured at day 1, 2, 7, and 20 posttransplantation, respectively. Overall, ASC-in-fused mice showed statisticalliy significant decrease in serum IL-6 levels than PBS-infused mice did throughout all posttransplantation periods. a P<0.005. |
 | Fig. 3.Representative photographs which demonstrate a mouse skin allograft specimen containing 4’,6-diamidino-2-phenyl-indole (DAPI)-labeled human adipose-derived stem cells. (A) After obtaining the skin graft at 7-posttransplantation day, (B) the frozen section of the tissue was observed under a fluo-rescent microscope (×100). The staining with DAPI released cy-an fluorescence. |
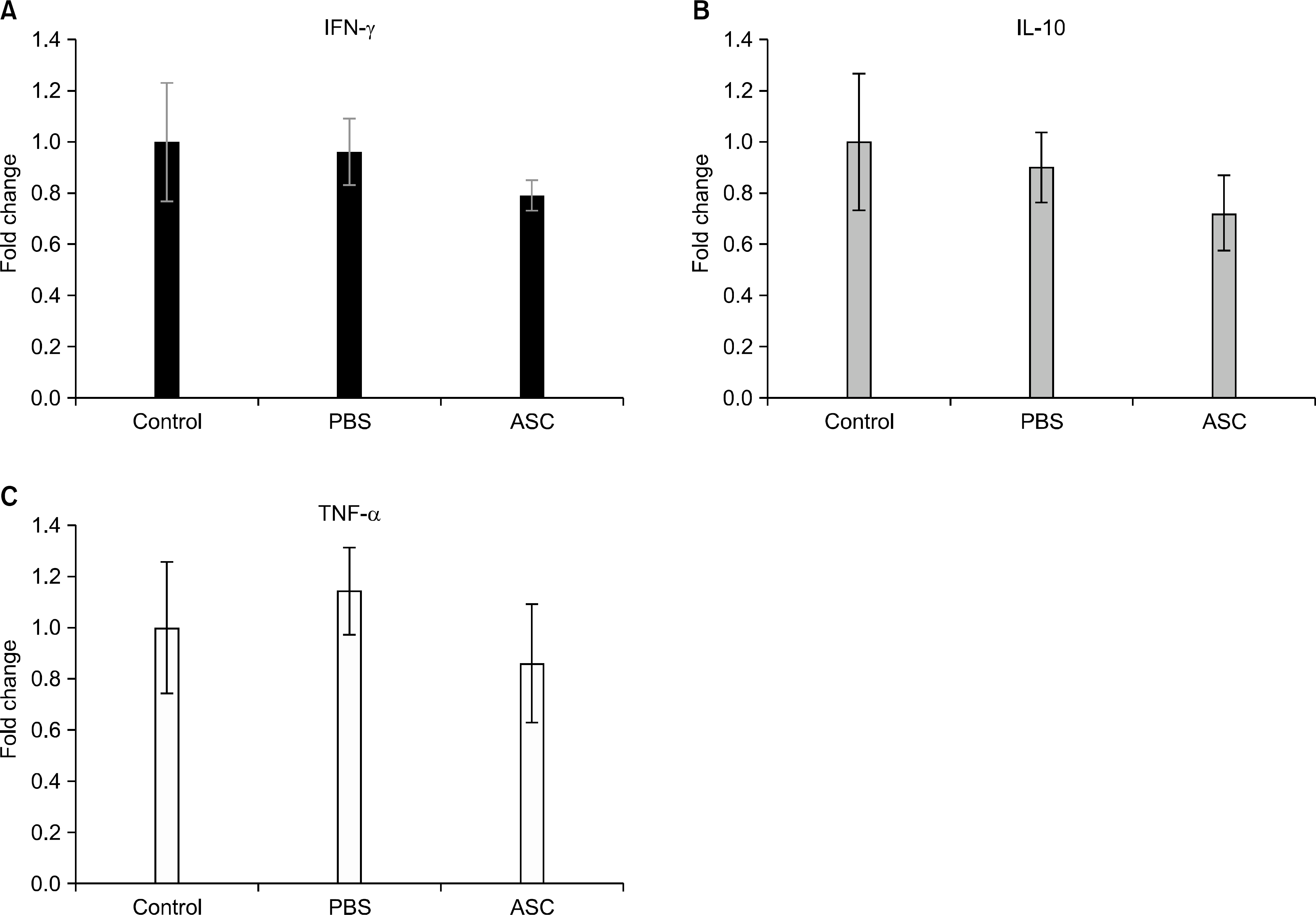 | Fig. 4.mRNA expressions of proinflammatory (A) interferon (IFN)-γ , (B) interleukin (IL)-10, and (C) tumor necrosis factor (TNF)-α genes in the skin allografts at day 7 posttransplantation manifested by real-time polymerase chain reaction. The mRNA expressions in phosphate buffered saline (PBS) (n=8) and adipose tissue-derived stem cell (ASC) (n=8) were expressed as fold changes in relation to the control group (n=8). The expressions of IFN-γ, IL-2, and TNF-α gene mRNA more decreased in ASC-infused mice than they did in PBS-in-fused mice, though the difference did not reach statistical significance. |
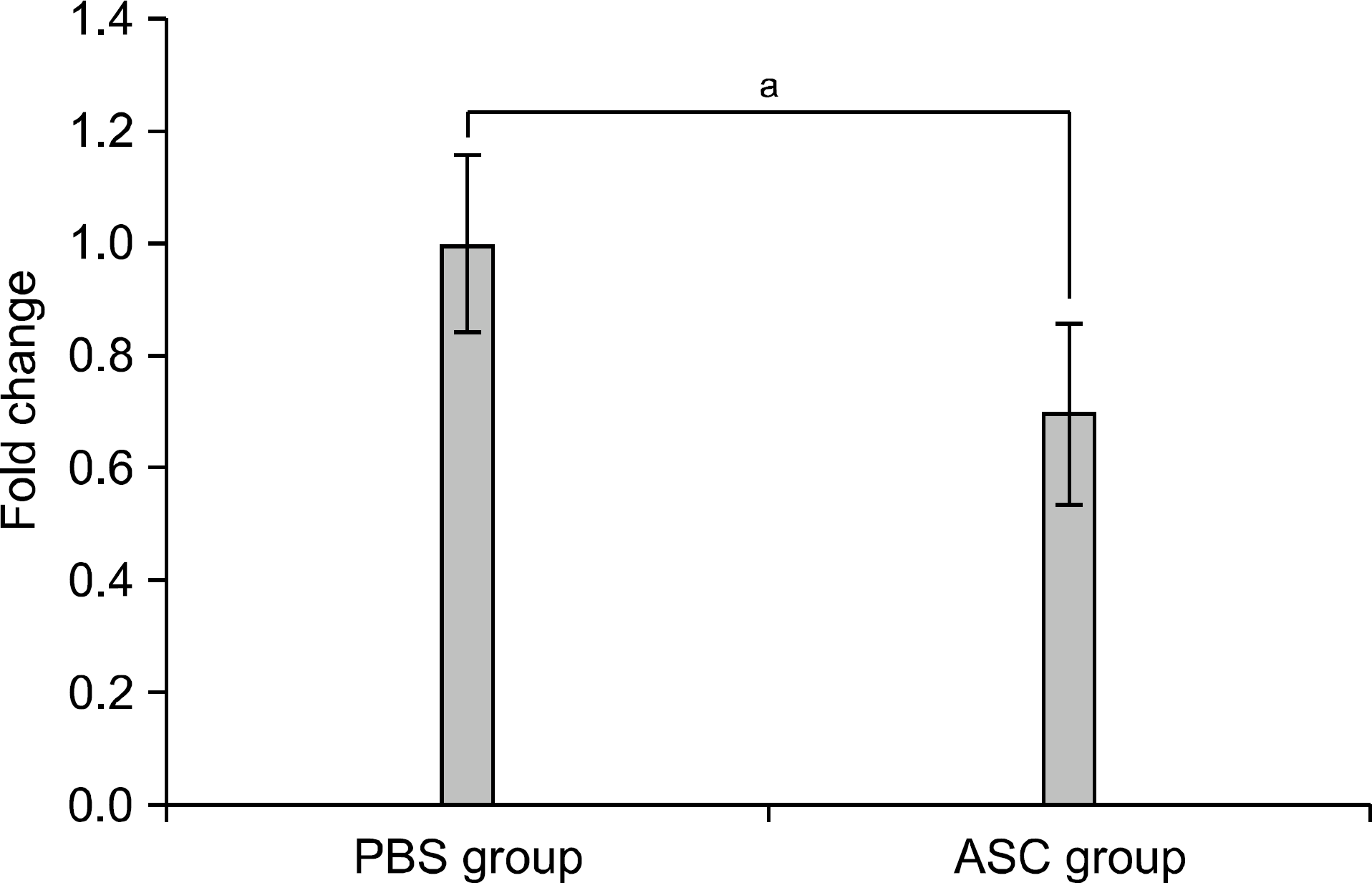 | Fig. 5.Mixed lymphocyte reaction at day 30 posttransplantation. Data are expressed as fold changes related to the phosphate buffered saline group. The alloreactivity between res-ponder cells (from BALB/c mice of corresponding groups) and stimulating cells (from C57BL/6 mice) was measured using CCK assay. Adipose tissue derived stem cell (ASC) group (n=8) showed significantly reduced alloreactivity compared with PBS group (n=8) (1.00±0.16 vs. 0.70±0.16; P=0.002). a P=0.002. |
 | Fig. 6.Representative microphotographs comparing skin allograft specimens between phosphate buffered saline (PBS)-infused mice and adipose tissue-derived stem cell (ASC)-infused mice after skin allograft transplantation. (A) At day 7 posttransplantation, the inflammatory reactions, which had been prominent in PBS-infusion group on day 7 posttransplantation, attenuated after ASC infusion on HE stain (Aa, Ab). Vasoactive endothelial growth factor (VEGF) was minimally or not expressed in PBS group, but its expression was enhanced in ASC-infused group (Ac, Ad). (B) At day 20 posttransplantation, most of full-thickness skin grafts treated with PBS were detached from the recipient’s tissue (Ba). However, the considerable number of grafts treated with ASC was persisted with limited inflammatory cell infiltration (Bb). And, VEGF was lesser expressed on day 20 posttransplantation than it did on day 7 posttransplantation in ASC-infused group (Bc, Bd). |
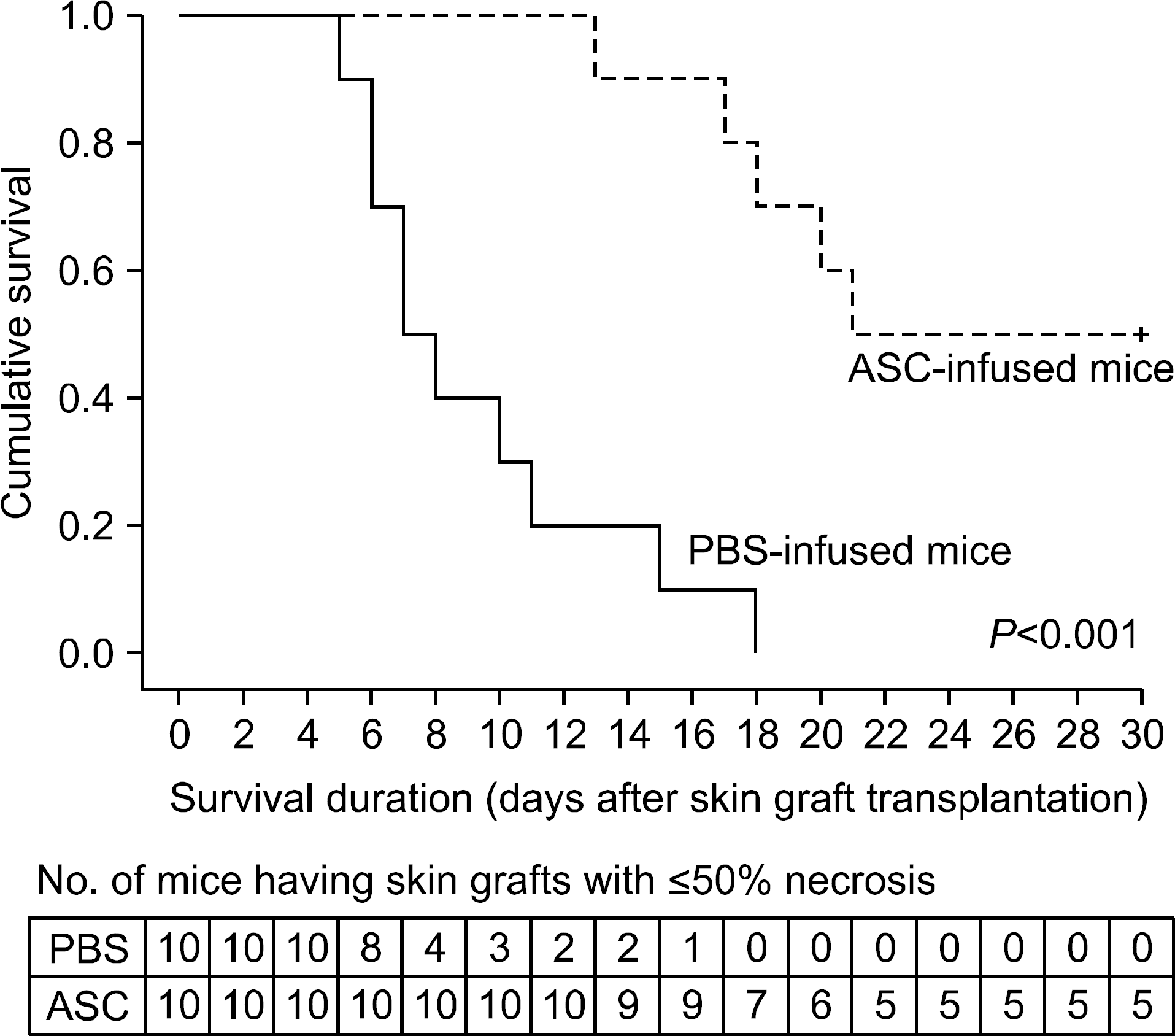 | Fig. 7.Kaplan-Meier allograft survival curves for mice which had been intravenously administrated phosphate buffered saline (PBS) and adipose tissue-derived stem cells (ASC; 1x106). Each group included 10 mice. ASC infusion markedly increased skin allograft survival in comparison with PBS infusion (P<0.001). |
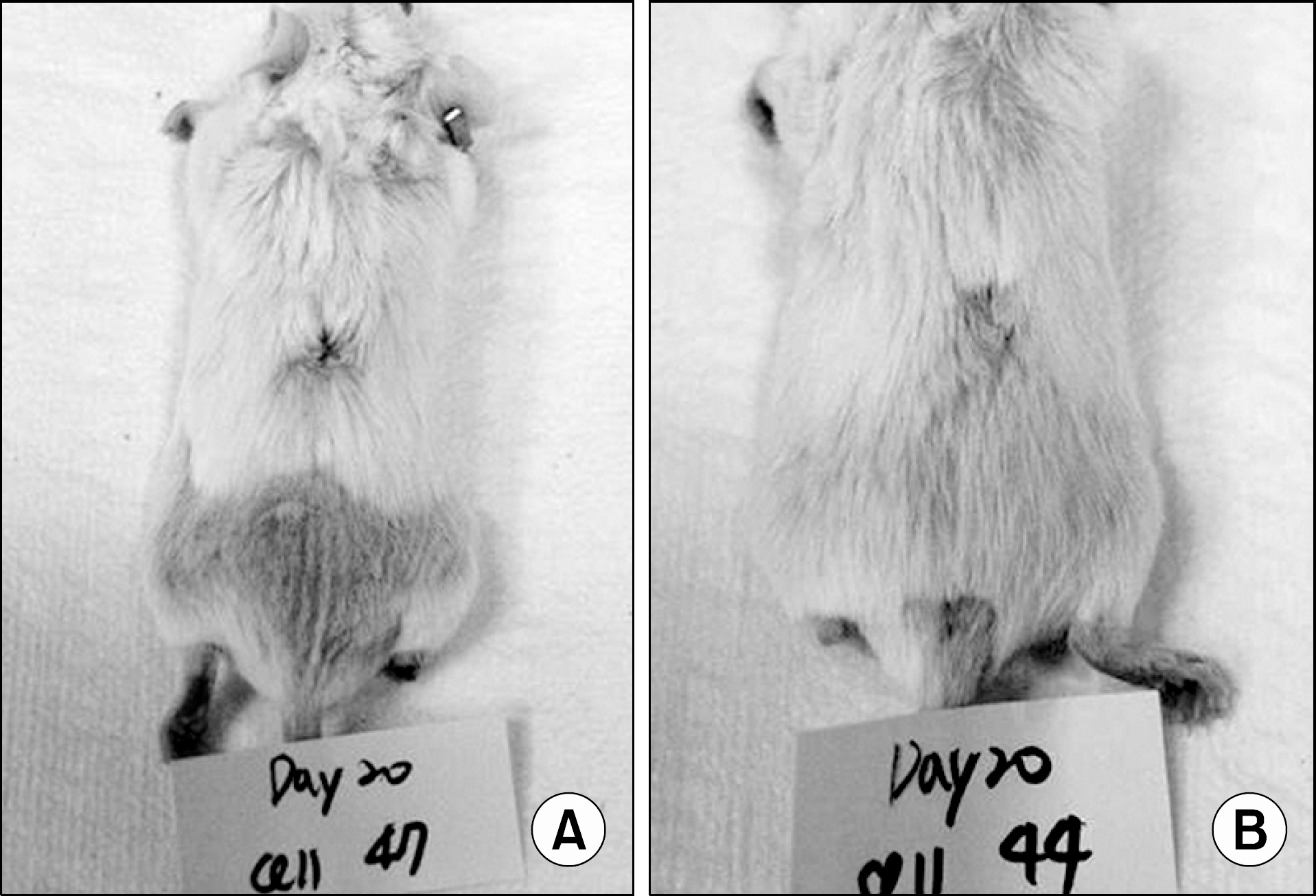 | Fig. 8.Representative photographs of skin allografts showing skin allograft acceptance. Two adipose tissue-derived stem cells (ASC)-infused mice (A, B) showed necrosis-free skin graft suggestive of graft acceptance at day 20 posttransplantation, showing the role of ASC inducing immunological tolerance. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download