Abstract
Antiphospholipid syndrome nephropathy (APSN) is well documented in the literature as the renal involvement of the antiphospholipid syndrome (APS). A review of literature also shows that among antiphospholipid antibodies, lupus anticoagulant (LA) positivity is recognized as the strongest risk factor for APSN. In addition, APSN is also known to be associated with a poor functional outcome in the first posttransplant year. Therefore, it is a general belief that renal transplantation may be life threatening in APS patients. Furthermore, the presence of LA at the time of transplantation is particularly associated with a high rate of allograft APSN and the consequent poor transplantation outcomes. Here, we report the case that thrombotic acute kidney injury due to APSN after kidney transplantation can be successfully treated if anticoagulation therapy is timely applied with a prompt diagnosis.
REFERENCES
2). Uthman I, Khamashta M. Antiphospholipid syndrome and the kidneys. Semin Arthritis Rheum. 2006; 35:360–7.

3). Hadaya K, Ferrari-Lacraz S, Fumeaux D, Boehlen F, Toso C, Moll S, et al. Eculizumab in acute recurrence of thrombotic microangiopathy after renal transplantation. Am J Transplant. 2011; 11:2523–7.

4). Canaud G, Bienaimé F, Noël LH, Royal V, Alyanakian MA, Dautzenberg MD, et al. Severe vascular lesions and poor functional outcome in kidney transplant recipients with lupus anticoagulant antibodies. Am J Transplant. 2010; 10:2051–60.

5). Tektonidou MG, Sotsiou F, Nakopoulou L, Vlachoyia-nnopoulos PG, Moutsopoulos HM. Antiphospholipid syndrome nephropathy in patients with systemic lupus eryth-ematosus and antiphospholipid antibodies: prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 2004; 50:2569–79.

6). Dolff S, Berden JH, Bijl M. Treatment of lupus nephritis. Expert Rev Clin Immunol. 2010; 6:901–11.

7). Ripert T, Menard J, Schoepen Y, N'Guyen P, Rieu P, Brandt B, et al. Anticoagulation after renal transplantation: a multicenter survey on clinical practice. Prog Urol. 2009; 19:186–91.
8). Rubenwolf P, Lopau K, Gerharz EW, Heidbreder E, Riedmiller H. Antiphospholipid antibody syndrome: a pri-ori a contraindication to kidney transplantation? Aktuelle Urol. 2007; 38:132–6.
Fig. 1.
Echocardiogram showing right atrium thrombus. After re-moval via submammrial incision, thrombus was disappeared.
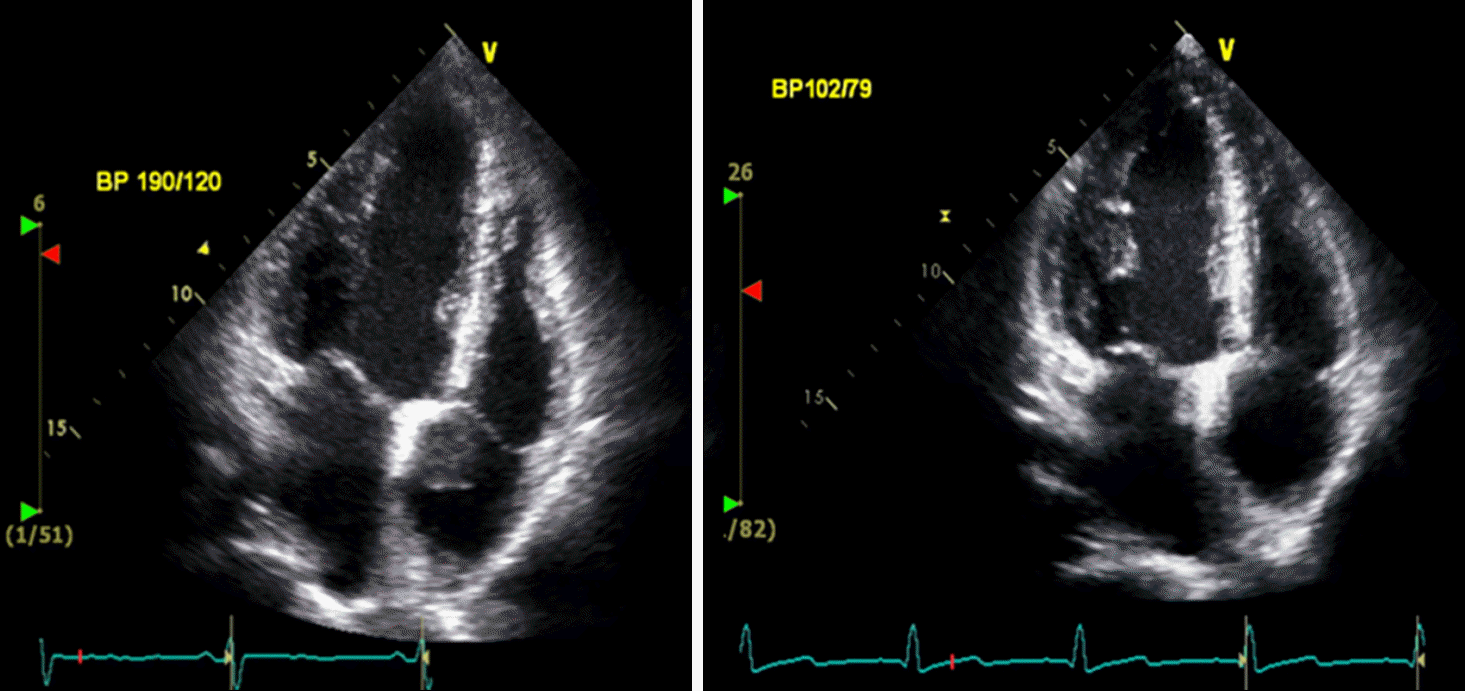
Fig. 2.
The biopsy slide of transplanted kidney showing cortical necrosis, focal (postoperative day 8) (A, HE stain, ×120; B, PAS stain,×120).
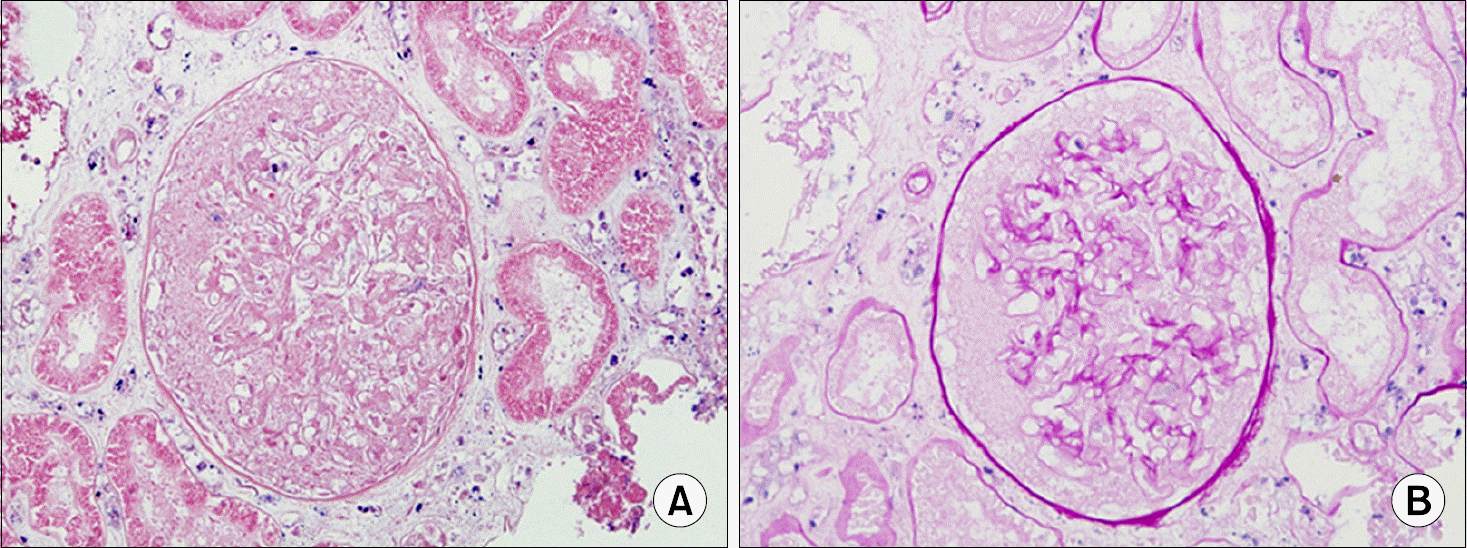
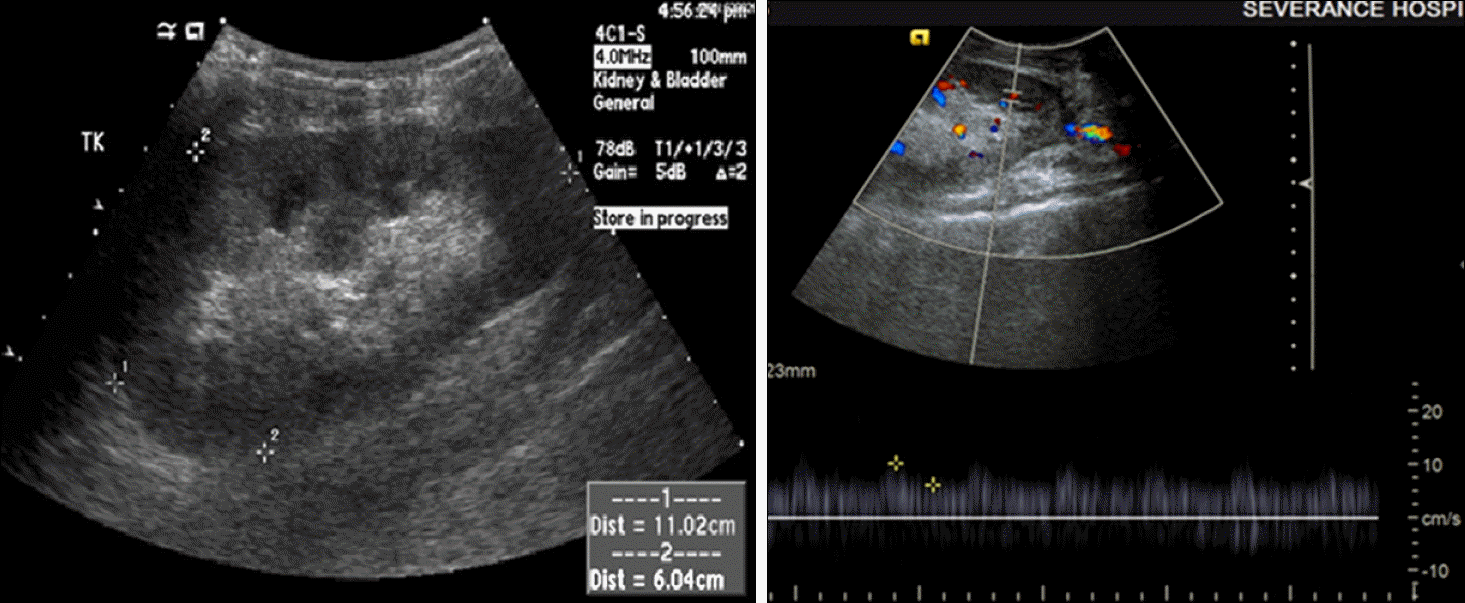
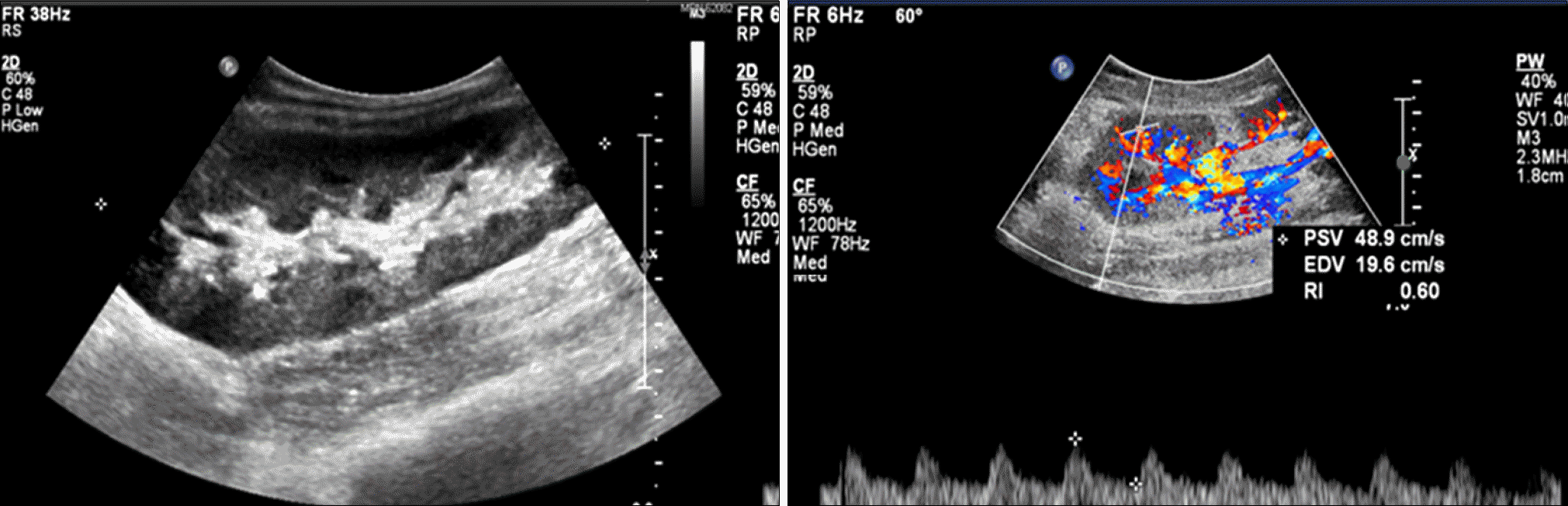
Fig. 3.
Sonography of transplanted kidney (postoperative day 15). The transplanted kidney size was 11.4 cm. The echogenicity of the cortex was increased and blood flow was decreased. The resistive index of upper, mid, and lower pole was 0.56.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download