Abstract
Background:
The occurrence of malignancy following kidney transplantation has been estimated three to five times the incidence compared to that of the general population. It is estimated that particularly in renal cell carcinoma (RCC), the relative risk increases. The aim of this study was to analyze the characteristics, risk factors, and prognosis of RCC following kidney transplantation.
Methods:
Total number of 3,272 kidney recipients who underwent transplantation from April 1979 to December 2012 and patients who had RCC following kidney transplantation were retrospectively reviewed and analyzed.
Results:
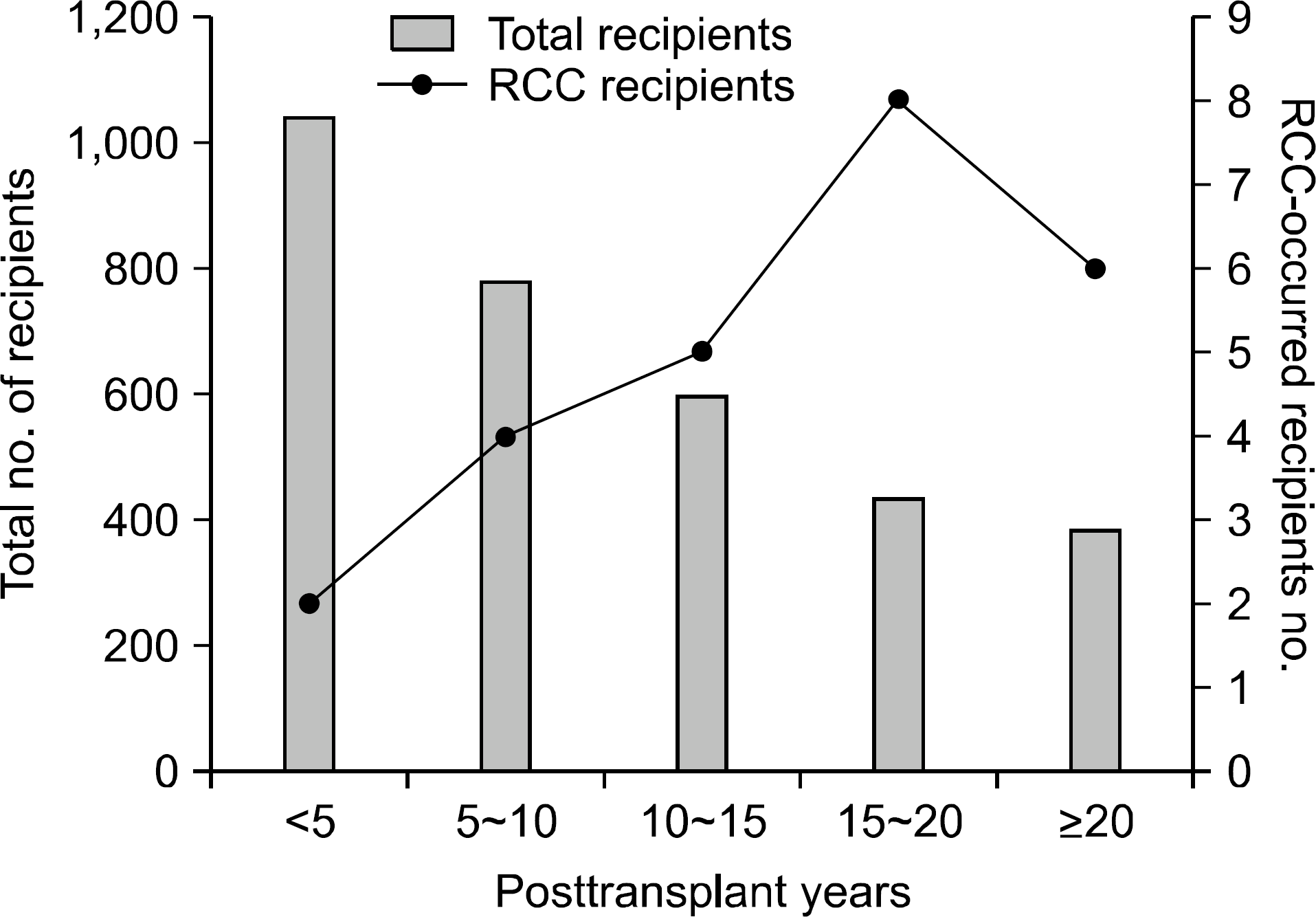
We found that among 232 cases of posttransplant malignancies, 25 recipients were diagnosed with RCC. We have observed in our study that it took an average of 175.2±71.0 months to develop RCC after their first kidney transplantation. However, with longer follow up period, interval incidence of RCC increased. Fourteen patients (56%) were diagnosed with RCC 15 years after transplantation. We also found that with reference to the risk factor analysis for posttransplant RCC, the long-term follow-up period was the only independent risk factor. In our study, 21 patients with RCC were treated with radical nephrectomy. Of them, 16 patients survived, and four RCC-related deaths occurred. Furthermore, the patient survival rate of RCC recipients was lower than that of the nonmalignancy group despite the graft survival rate were not different.
Go to : 
REFERENCES
1). Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009; 69:2227–43.
2). Ju MK, Joo DJ, Kim SJ, Huh KH, Kim MS, Jeon KO, et al. Chronologically different incidences of posttransplant malignancies in renal transplant recipients: single center experience. Transpl Int. 2009; 22:644–53.

3). Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005; 80(2 Suppl):S254–64.

4). Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004; 4:905–13.

5). Leveridge M, Musquera M, Evans A, Cardella C, Pei Y, Jewett M, et al. Renal cell carcinoma in the native and allograft kidneys of renal transplant recipients. J Urol. 2011; 186:219–23.

6). Végsö G, Toronyi E, Hajdu M, Piros L, Görög D, Deák PA, et al. Renal cell carcinoma of the native kidney: a frequent tumor after kidney transplantation with favor-able prognosis in case of early diagnosis. Transplant Proc. 2011; 43:1261–3.

7). Zafar SY, Howell DN, Gockerman JP. Malignancy after solid organ transplantation: an overview. Oncologist. 2008; 13:769–78.

8). Doublet JD, Peraldi MN, Gattegno B, Thibault P, Sraer JD. Renal cell carcinoma of native kidneys: prospective study of 129 renal transplant patients. J Urol. 1997; 158:42–4.

9). Schwarz A, Vatandaslar S, Merkel S, Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007; 2:750–6.

10). Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1994; 1994:99–109.
11). Ianhez LE, Lucon M, Nahas WC, Sabbaga E, Saldanha LB, Lucon AM, et al. Renal cell carcinoma in renal transplant patients. Urology. 2007; 69:462–4.

12). Heinz-Peer G, Schoder M, Rand T, Mayer G, Mostbeck GH. Prevalence of acquired cystic kidney disease and tu-mors in native kidneys of renal transplant recipients: a prospective US study. Radiology. 1995; 195:667–71.

13). Hoshida Y, Nakanishi H, Shin M, Satoh T, Hanai J, Aozasa K. Renal neoplasias in patients receiving dialysis and renal transplantation: clinico-pathological features and p53 gene mutations. Transplantation. 1999; 68:385–90.

14). Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveil-lance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003; 30:843–52.

15). Kasiske BL, Vazquez MA, Harmon WE, Brown RS, Danovitch GM, Gaston RS, et al. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000; 11(Suppl 15):S1–86.
Go to : 
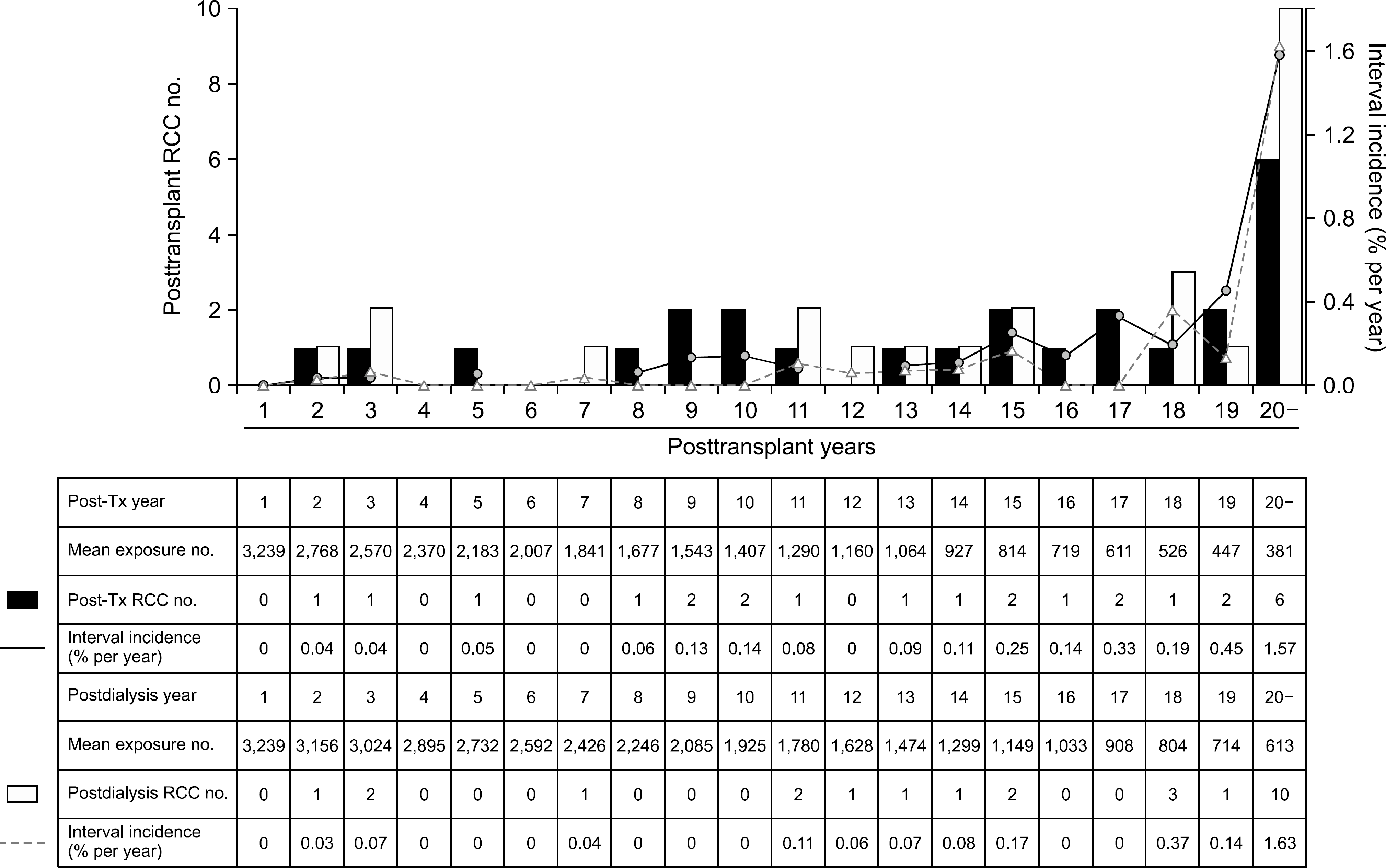 | Fig. 1.Interval incidence of renal cell carcinoma after kidney transplantation. Interval incidence of posttransplant renal cell carcinoma (RCC) increased time-dependently. Also, as the duration from starting dialysis increased, interval incidence of postdialysis RCC increased. Abbreviation: Post-Tx, posttransplant. |
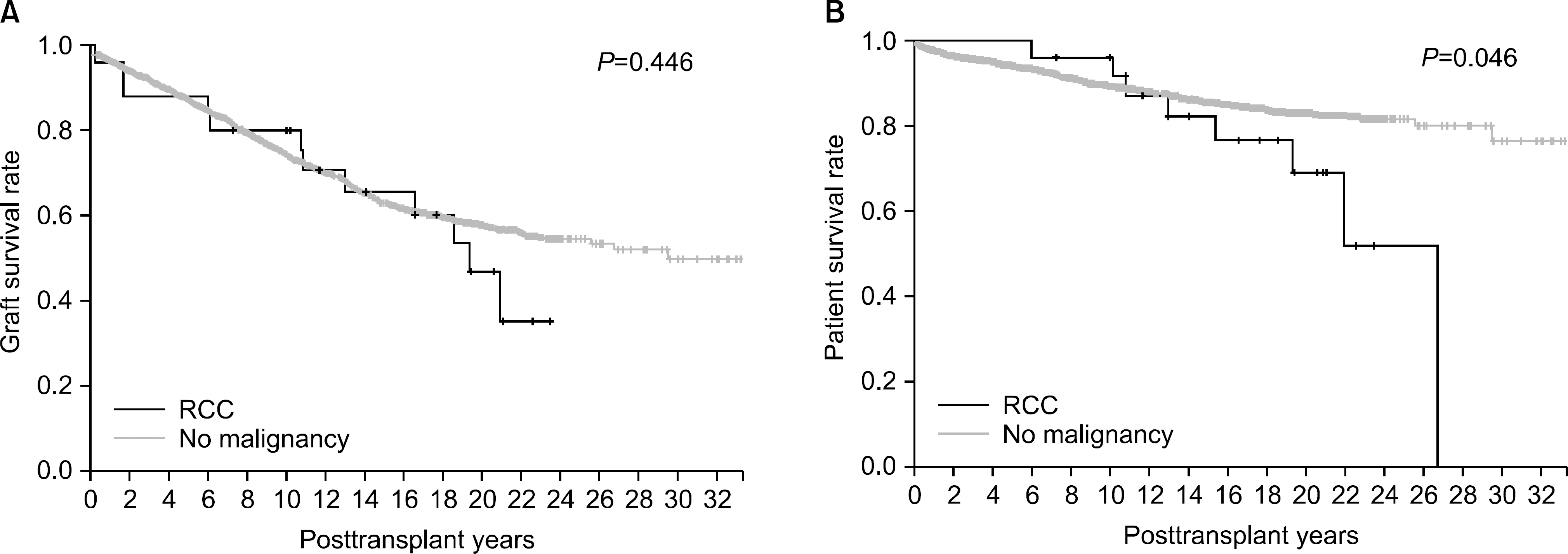 | Fig. 3.(A, B) Graft and patient survival rates. Patient survival rates of the renal cell carcinoma (RCC) patients were worse than the patients without malignancy but graft survival rates were not different between the two groups. |
Table 1.
Clinical manifestations of posttransplant renal cell carcinoma patients
| Characteristic | Value |
|---|---|
| Age at transplantation (yr) | 40.5±10.0 |
| Sex (male:female) | 19:6 |
| Donor age (yr) | 37.6±11.6 |
| Donor sex (male:female) | 14:11 |
| Recipient-donor relation | LRD (11)/LURD (11)/ Deceased (3) |
| Age at diagnosis of RCC (yr) | 58.4±10.8 |
| Mean interval between 1st transplantation and RCC (mo) | 175.2±71.0 (20∼269) |
| 2nd graft | 7 a |
| RCC site (right:left:transplant) | 12:11:2 |
| RCC pathology | |
| Clear cell type | 9 |
| Convention type | 2 |
| Cystic | 2 |
| Papillary | 6 |
| Chromophobe type | 1 |
| Sarcomatoid | 1 |
| Others | 4 |
| Mean survival after RCC treatment (mo) | 38.8±31.2 |
Table 2.
Comparison between renal cell carcinoma and nonmalignancy patients
Table 3.
Risk factor analysis for posttransplant renal cell carcinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download