Abstract
Background:
Lung transplantation (LTx) is an effective treatment for end stage lung disease. However, the shortage of donor lungs has been a major limiting factor to increase the number of LTx. Ex vivo lung perfusion (EVLP) is a currently approved method to evaluate lung function and to repair donor lung with poor function. The purpose of this study was to develop EVLP system in pig model and to maintain lung function during 4 hours of EVLP.
Methods:
Bilateral lung blocks were harvested from five 40 kg pigs. These blocks were applied in EVLP perfused with 37o
C Steen solution. We performed arterial blood gas (ABG) analyses before death and also every 1 hour for 4 hours after application of EVLP and calculated oxygen capacities (OC) using the results of ABG. We also calculated pulmonary vascular resistance (PVR) and peak airway pressure (PAP) every 1 hour for 4 hours. After EVLP procedure, we excised specimens for pathologic review.
Results:
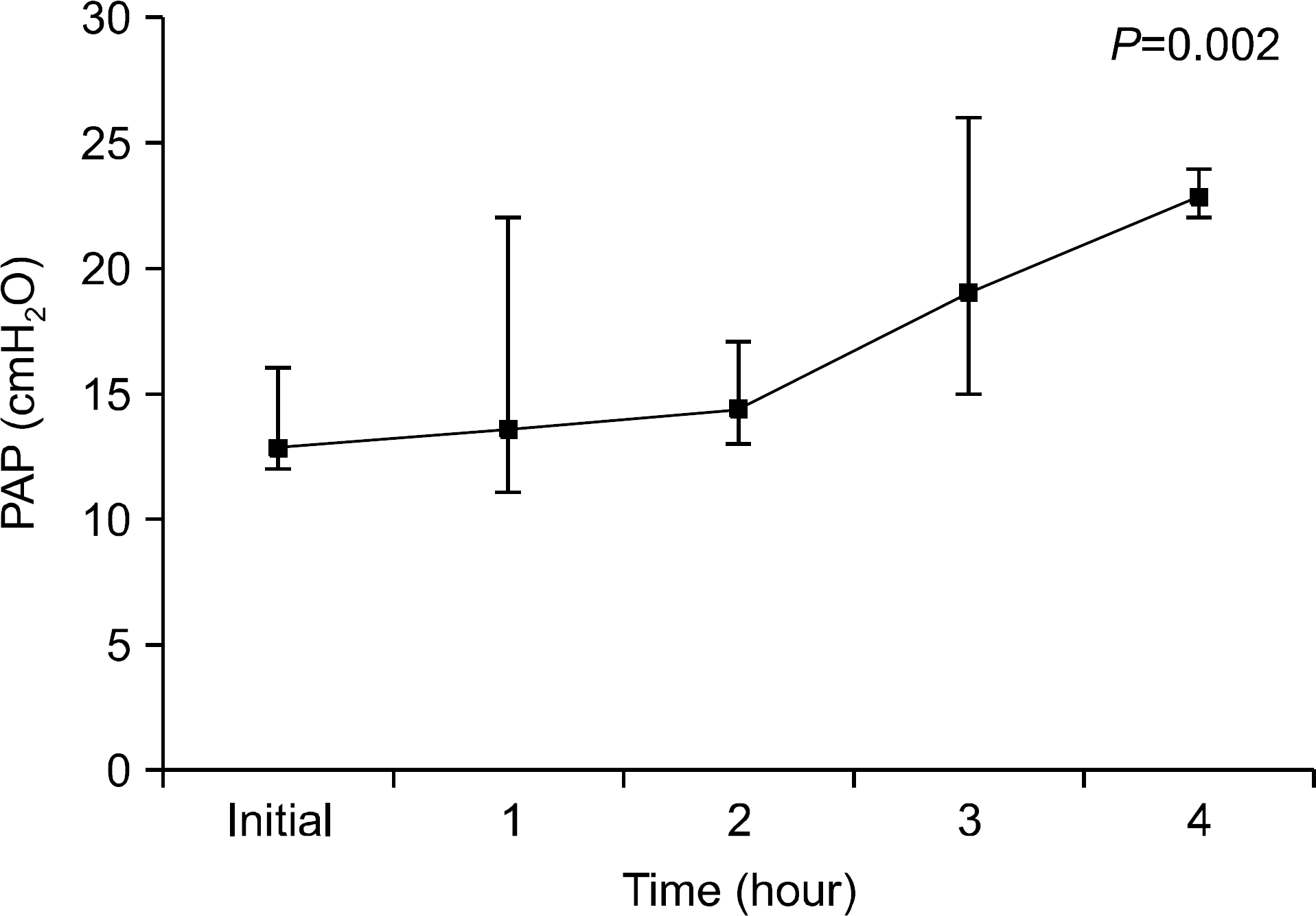
We found that OC gradually decreased during the 4 hour period of EVLP; however, no statistically significant difference was obtained. PVR declined sharply after 1 hour of EVLP (P=0.031) and then remained constant for 3 hours. PAP significantly increased after 3 hours (P<0.0001). Pathologic investigations revealed various findings from normal lung to severe pulmonary edema.
Go to : 
REFERENCES
1). Paik HC, Haam SJ, Lee DY, Yi GJ, Song SW, Kim YT, et al. The fate of patients on the waiting list for lung transplantation in Korea. Transplant Proc. 2012; 44:865–9.

2). Paik HC, Haam SJ, Lee DY, Yi GJ, Song SW, Kim YT, et al. Donor evaluation for lung transplantation in Korea. Transplant Proc. 2012; 44:870–4.

3). de Antonio DG, Marcos R, Laporta R, Mora G, García-Gallo C, Gámez P, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007; 26:529–34.

4). Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995; 46:235–47.
5). Cypel M, Rubacha M, Yeung J, Hirayama S, Torbicki K, Madonik M, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009; 9:2262–9.
6). Wisser W, Oturanlar D, Minich R, Ringl H, Wekerle T, Klepetko W, et al. Closed circuit perfusion of an isolated rabbit lung. A new model for the evaluation of preservation quality of stored lungs. Eur J Cardiothorac Surg. 1993; 7:71–4.
7). Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjöberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003; 76:244–52.

8). Koike T, Yeung JC, Cypel M, Rubacha M, Matsuda Y, Sato M, et al. Kinetics of lactate metabolism during acel-lular normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2011; 30:1312–9.

9). de Perrot M, Snell GI, Babcock WD, Meyers BF, Patterson G, Hodges TN, et al. Strategies to optimize the use of currently available lung donors. J Heart Lung Transplant. 2004; 23:1127–34.

10). Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, et al. Ex vivo evaluation of non-acceptable donor lungs. Ann Thorac Surg. 2006; 81:460–6.

11). Egan TM, Haithcock JA, Nicotra WA, Koukoulis G, Inokawa H, Sevala M, et al. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006; 81:1205–13.

12). Sanchez PG, Bittle GJ, Burdorf L, Pierson RN 3rd, Griffith BP. State of art: clinical ex vivo lung perfusion: rationale, current status, and future directions. J Heart Lung Transplant. 2012; 31:339–48.

13). Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008; 27:1319–25.

14). Watanabe N, Sakota D, Ohuchi K, Takatani S. Deforma-bility of red blood cells and its relation to blood trauma in rotary blood pumps. Artif Organs. 2007; 31:352–8.

15). Hopkinson DN, Bhabra MS, Odom NJ, Bridgewater BJ, Van Doorn CA, Hooper TL. Controlled pressure re-perfusion of rat pulmonary grafts yields improved function after twenty-four-hours' cold storage in University of Wisconsin solution. J Heart Lung Transplant. 1996; 15:283–90.
16). Wierup P, Liao Q, Bolys R, Sjöberg T, Rippe B, Steen S. Lung edema formation during cold perfusion: im-portant differences between rat and porcine lung. J Heart Lung Transplant. 2005; 24:379–85.

17). Myrianthefs PM, Briva A, Lecuona E, Dumasius V, Rutschman DH, Ridge KM, et al. Hypocapnic but not metabolic alkalosis impairs alveolar fluid reabsorption. Am J Respir Crit Care Med. 2005; 171:1267–71.

18). Petak F, Habre W, Hantos Z, Sly PD, Morel DR. Effects of pulmonary vascular pressures and flow on airway and parenchymal mechanics in isolated rat lungs. J Appl Physiol. 2002; 92:169–78.
19). Broccard AF, Vannay C, Feihl F, Schaller MD. Impact of low pulmonary vascular pressure on ventilator-induced lung injury. Crit Care Med. 2002; 30:2183–90.

Go to : 
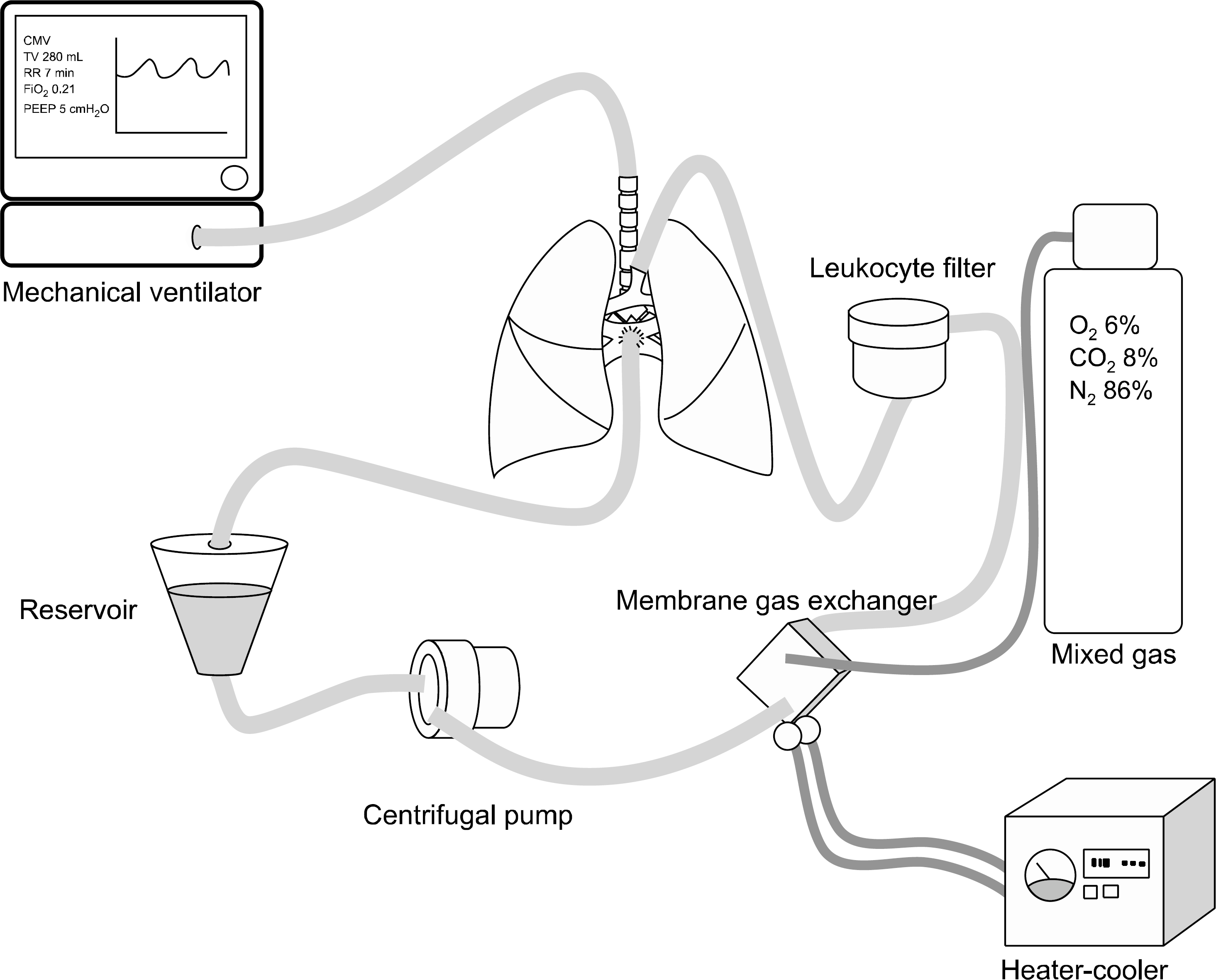 | Fig. 1.Diagram of ex vivo lung perfusion model. This model con-sists of mechanical ventilator, res-ervoir, centrifugal pump, membrane gas exchanger, and leukocyte filter. A heater-cooler main-tains the perfusate at 37 o C. A mix gas is connected to membrane gas exchanger. |
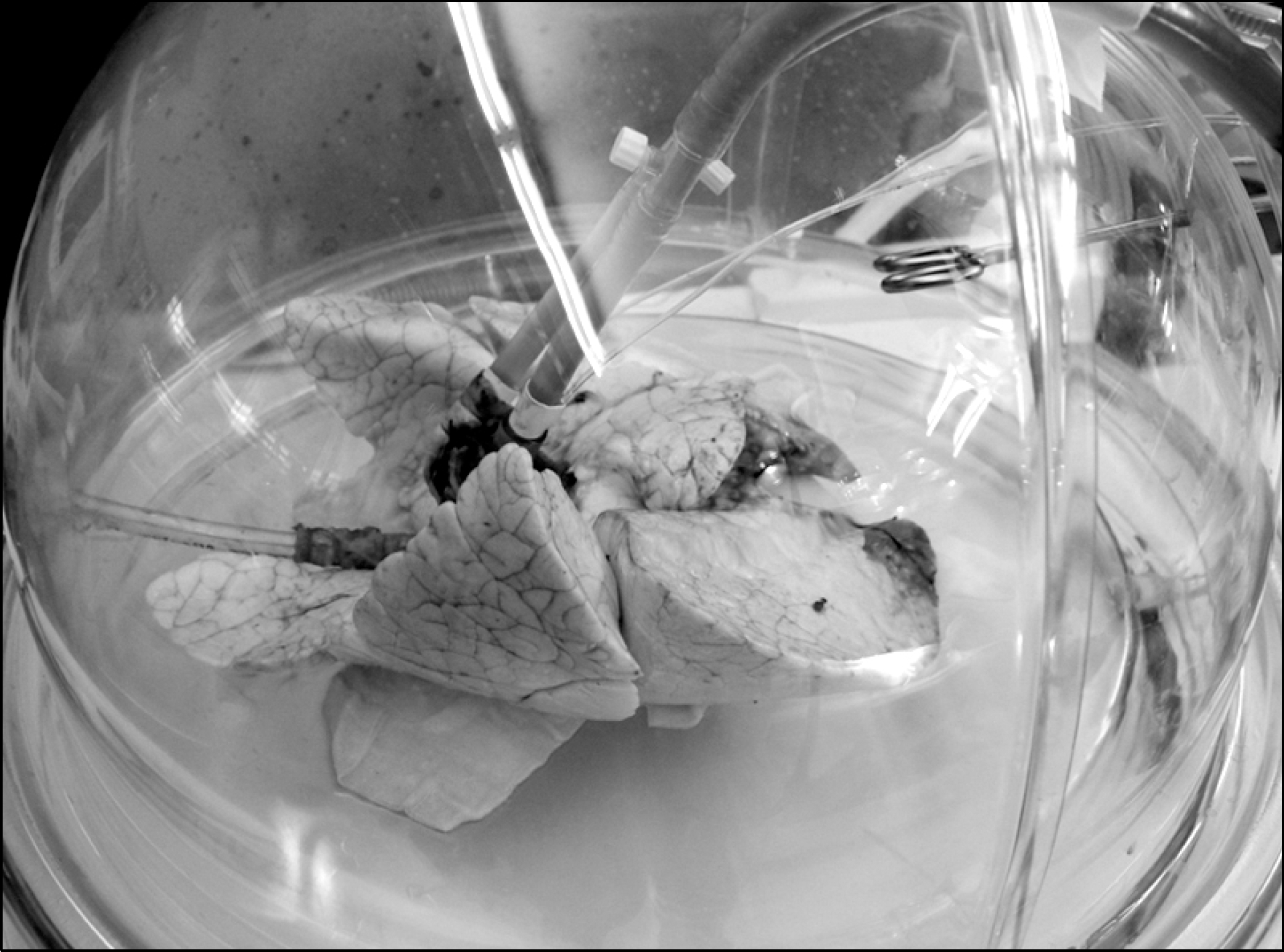 | Fig. 2.Funnel shaped catheter was sutured to left atrium and pulmonary artery catheter was inserted to main pulmonary artery. Endotracheal intubation tube was inserted to trachea. Donor lung was preserved in dom shaped container during ex vivo lung perfusion. |
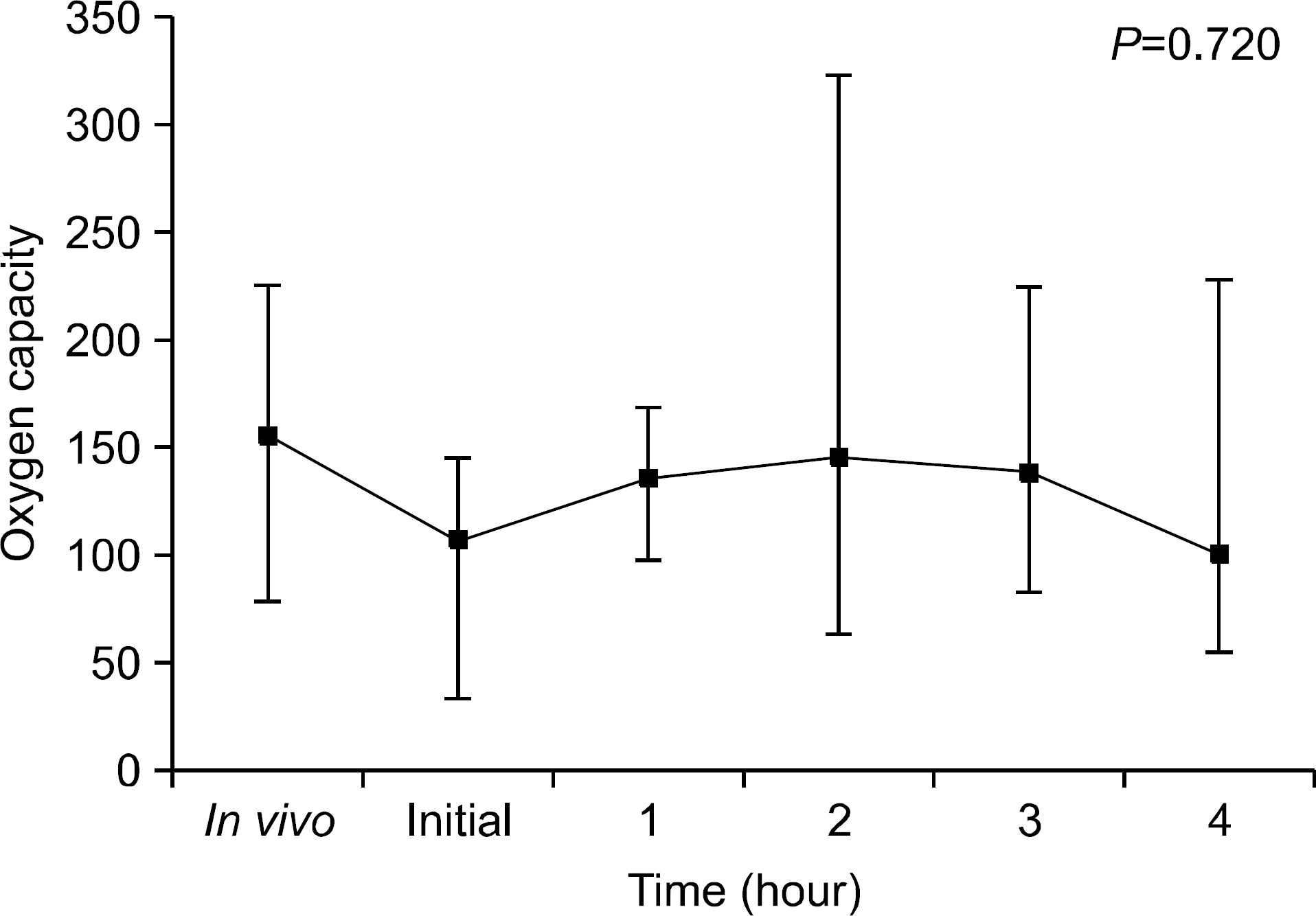 | Fig. 3.Oxygen capacity during ex vivo lung perfusion (EVLP). Oxygen capacities gradually decreased after start of EVLP, but these were not statistically significant. |
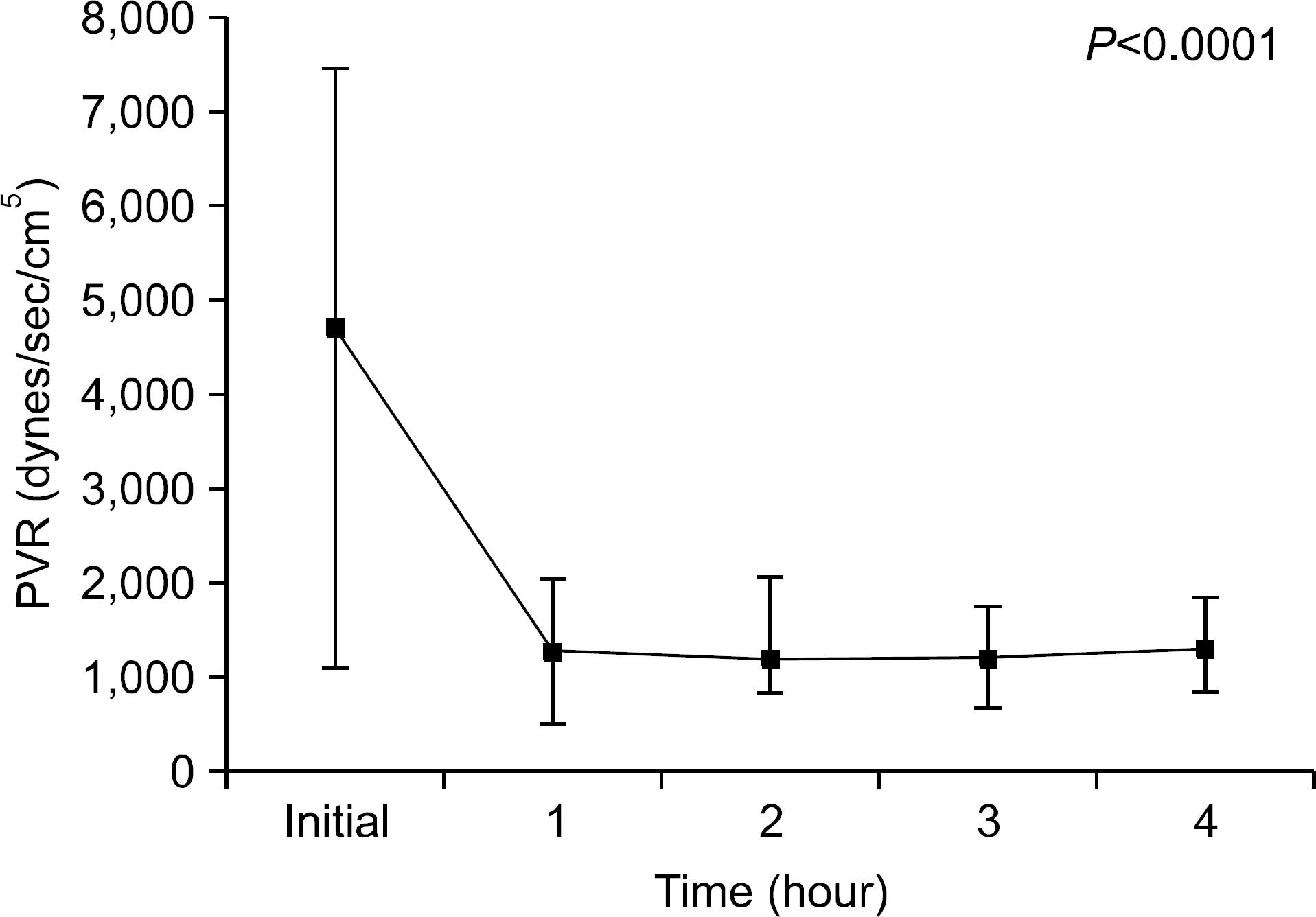 | Fig. 4.Pulmonary vascular resistance during ex vivo lung perfusion (EVLP). Pulmonary vascular resistance (PVR) declined sharply after 1 hour of EVLP application (P=0.031), and these were consistently maintained for 3 hours. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download