Abstract
Background
Everolimus and cyclosporine (CsA) exhibit synergistic immunosuppressive activity when used in combination. We analyzed preliminary data about the use of everolimus with a CsA-sparing strategy in de novo renal transplant recipients.
Methods
A comparative, parallel, randomized, open-label, 1 year study has been performed in 117 patients from 5 transplant centers to compare the efficacy and tolerability of everolimus (EVE)+reduced-dose CsA or enteric-coated mycophenolate sodium (Myfortic)+standard-dose CsA in combination with basiliximab and steroids. It ended on August 24, 2011. Efficacy failure (biopsy-proven acute rejection, death, graft loss, or loss to follow-up), safety, and renal function were evaluated at 1, 3, 5, and 12 months post-transplantation.
Results
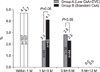
Efficacy failure was comparable between the two groups. Only one graft loss has been reported in the control group and no patient death reported in either group. There was no significant difference in the incidence of biopsy-proven acute rejection until 3 and 5 month post-transplantation (P>0.05). The mean e-GFR of the group of EVE+reduced-dose CsA was significantly higher than that of the control group at 3 (65.6±16.9 mL/mim/1.73 m2 vs. 56.7±14.4 mL/mim/1.73 m2; P=0.007) and 5 (68.6±18.8 mL/mim/1.73 m2 vs. 58.1±16.2 mL/mim/1.73 m2; P=0.009) months. There was no significant difference in the incidence of discontinuations and serious adverse events between the groups (P>0.05).
Figures and Tables
References
2. Meier-Kriesche KU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004. 4:1289–1295.

3. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003. 349:2326–2333.

4. Nankivell BJ, Bowwors RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RDM. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004. 78:557–565.

5. Moien-Afshari F, McManus BM, Laher I. Immunosuppression and transplant vascular disease: benefits and adverse effects. Pharmacol Ther. 2003. 100:141–156.

6. Weir MR, Ward MT, Blahut SA, Klassen DK, Cangro CB, Bartlett ST, et al. Long-term impact of discontinued or reduced calcineurin inhibitor in patients with chronic allograft nephropathy. Kidney Int. 2001. 59:1567–1573.

7. Neumayer HH. Introducing everolimus (Certican) in organ transplantation: an overview of preclinical and early clinical developments. Transplantation. 2005. 79:9 Suppl. S72–S75.

8. Pape L, Ahlenstiel T, Ehrich JH, Offner G. Reversal of loss of glomerular filtration rate in children with transplant nephropathy after switch to everolimus and low-dose cyclosporine A. Pediatr Transplant. 2007. 11:291–295.

9. Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008. 22:1–15.

10. Hueso M, Bover J, Serón D, Gil-Vernet S, Sabaté I, Fulladosa X, et al. Low-dose cyclosporine and mycophenolate mofetil in renal allograft recipients with suboptimal renal function. Transplantation. 1998. 66:1727–1731.

11. Kerecuk L, Taylor J, Clark G. Chronic allograft nephropathy and mycophenolate mofetil introduction in paediatric renal recipients. Pediatr Nephrol. 2005. 20:1630–1635.

12. Pascual M, Curtis J, Delmonico FL, Farrell ML, Williams WW Jr, Kalil R, et al. A prospective, randomized clinical trial of cyclosporine reduction in stable patients greater than 12 months after renal transplantation. Transplantation. 2003. 75:1501–1505.

13. Ciancio G, Burke GW, Gaynor JJ, Ruiz P, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus/sirolimus versus tacrolimums/mycophenolate versus cyclosporine/sirolimus in renal transplantation: three-year analysis. Transplantation. 2006. 81:845–852.

14. Formica RN Jr, Lorber KM, Friedman AL, Bia MJ, Lakkis F, Smith JD, et al. Sirolimus-based immunosuppression with reduce dose cyclosporine or tacrolimus after renal transplantation. Transplant Proc. 2003. 35:3 Suppl. 95S–98S.

15. Strom T, Haschke M, Zhang YL, Bendrick-Peart J, Boyd J, Roberts M, et al. Identification of everolimus metabolite patterns in trough blood samples of kidney transplant patients. Ther Drug Monit. 2007. 29:592–599.

16. Morales J, Fierro A, Benavente D, Zehnder C, Ferrario M, Contreras L, et al. Conversion from a calcineurin inhibitor-based immunosuppressive regimen to everolimus in renal transplant recipients: effect on renal function and proteinuria. Transplant Proc. 2007. 39:591–593.

17. Sánchez Fructuoso A, Ruiz San Millán JC, Calvo N, Rodrigo E, Moreno MA, Cotorruelo J, et al. Evaluation of the efficacy and safety of the conversion from a calcineurin inhibitor to an everolimus-based therapy in maintenance renal transplant patients. Transplant Proc. 2007. 39:2148–2150.

18. Pascual J. Concentration-controlled everolimus (Certican): combination with reduced dose calcineurin inhibitors. Transplantation. 2005. 79:9 Suppl. S76–S79.

19. Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation. 2005. 80:244–252.

20. Vitko S, Margreiter R, Weimar W, Dantal J, Viljoen HG, Li Y, et al. Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation. 2004. 78:1532–1540.

21. Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant. 2004. 4:626–635.

22. Kovarik JM, Tedesco H, Pascual J, Civati G, Bizot MN, Geissler J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced-exposure cyclosporine in de novo kidney transplantation. Ther Drug Monit. 2004. 26:499–505.

23. Tedesco-Silva H Jr, Vitko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12-month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transpl Int. 2007. 20:27–36.

24. Pietruck F, Budde K, Salvadori M, Sollinger H, Bourbigot B, Gentil MA, et al. Efficacy and safety of enteric-coated mycophenolate sodium in renal transplant patients with diabetes mellitus: post hoc analyses from three clinical trials. Clin Transplant. 2007. 21:117–125.

25. Carmellini M, Collini A, Ruggieri G, Garosi G, Bernini M. Excellent long-term results in de novo renal transplant recipients treated with proliferation signal inhibitors and reduced calcineurin inhibitors exposure. Transplant Proc. 2008. 40:1858–1861.

26. Budde K, Neumayer HH, Lehne G, Winkler M, Hauser IA, Lison A, et al. Tolerability and steady-state pharmacokinetics of everolimus in maintenance renal transplant patients. Nephrol Dial Transplant. 2004. 19:2606–2614.

27. Bouzas L, Tutor JC. Determination of everolimus in whole blood using the Abbott IMx sirolimus microparticle enzyme immunoassay. Clin Biochem. 2007. 40:132–136.

28. Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999. 67:1036–1042.

29. Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000. 69:1252–1260.

30. Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation. 2002. 74:1070–1076.

31. Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus-based calcineurin inhibitor-sparing and calcineurin inhibitor-free regimens in cadaveric renal transplantation. Transplantation. 2004. 77:1228–1235.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download