Abstract
The development of immunosuppressant treatments has enabled remarkable progress in the tissue and organ transplantation field by helping to prevent acute graft rejection. However, complications related to transplantation, such as infection by bacteria and viruses, and the occurrence of cancers resulting from prolonged immune suppression are major obstacles to overcome. Therefore, transplantation immunology research efforts should focus on the induction of donor-specific immune tolerance which preserves patient immune competence which promotes infection and cancer surveillance. Additionally, lifelong administration of immunosuppressants should be forgone in preference to short term therapies. In the 1990s, Dr. Shimon Sakaguchi identified the CD4+CD25+ regulatory T cells which develop in the thymus, and demonstrated that these cells play crucial roles in the maintenance of immune self tolerance. Studies which followed proved that these regulatory T cells are important to the control of autoimmune disease and prevention of graft rejection. Regulatory T cells have also been found to induce immune tolerance in rodent models. In this review, we discuss several considerations for the use of regulatory T cell therapy in the clinical transplantation field.
Figures and Tables
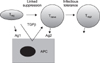 | Fig. 1Linked suppression and infectious tolerance. Linked suppression of naive T cells responding to antigen 2 (Ag2) can occur when the antigen-presenting cell (APC) is simultaneously also presenting a different antigen (Ag1) to regulatory T cells (Tregs). The Treg can then act to inhibit the full activation of the naive T cell, either because the naive T cell is brought within the range of close acting cytokines or cell surface ligands of the Treg or because the Treg can modify the status of the APC. One way in which Treg cells may modulate APC activity towards anti-inflammatory presentation is to produce transforming growth factor β (TGFβ). In some cases, the naive T cell may receive sufficiently tolerogenic signals from the Treg and/or APC that it is itself converted to a Treg cell. This second cohort of Tregs then confers infectious tolerance against Ag2. Reprinted from Fig. 1 of reference [86]. |
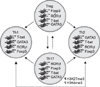 | Fig. 2Plasticity of CD4+ T Cells. Recent findings suggest that T helper cell differentiation is more plastic than previously appreciated. Each CD4+ T cell subset can adopt alternate cytokine profiles in response to cytokine environmental changes. Among four subsets of T cells, Treg cells and Th17 cells display the highest propensity to switch to other phenotypes. The molecular mechanism underlying this plasticity may be related to poised, bivalent epigenetic states (i.e., permissive H3K4me3 plus repressive H3K27me3 marks) at the transcriptional regulator (e.g., T-bet and Gata3) gene loci. Consistent with permissive epigenetic marks at Foxp3 and RORγt gene loci, coexpression of Foxp3 and RORγt occurs in Treg cells, but RORγt activity is inhibited by Foxp3. Reprinted from Fig. 2 of reference [49]. |
Table 1
Subsets of natural and induced regulatory T cellsa

aSubsets have been detected in humans and rodents. bIssue uncertain, not yet clear or not yet investigated.
Abbreviations: APC, antigen-presenting cell; DC, dendritic cell; ILT, immunoglobulin transcript; NKTreg, regulatory cell of natural killer T cell phenotype; Th3, T helper type 3; Tr1 cell, type 1 regulatory T cell; Treg, regulatory T cell.
Reprined from Table 1 of reference [85].
References
1. Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003. 3:199–210.

2. Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci U S A. 2004. 101:Suppl 2. 14622–14626.

3. Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004. 114:1398–1403.

4. Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007. 7:1457–1463.

5. Sagoo P, Lombardi G, Lechler RI. Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant. 2008. 13:645–653.

6. Waldmann H, Adams E, Fairchild P, Cobbold S. Regulation and privilege in transplantation tolerance. J Clin Immunol. 2008. 28:716–725.

7. Long E, Wood KJ. Regulatory T cells in transplantation: transferring mouse studies to the clinic. Transplantation. 2009. 88:1050–1056.

8. Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982. 156:1577–1586.

9. Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982. 156:1565–1576.

10. Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990. 172:1701–1708. Erratum in: J Exp Med 1991;173:1037.

11. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. 155:1151–1164.
12. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001. 27:20–21.

13. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.

14. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003. 4:330–336.

15. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003. 4:337–342.

16. Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003. 112:1437–1443.

17. Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997. 389:737–742.

18. Zelenika D, Adams E, Humm S, Graca L, Thompson S, Cobbold SP, et al. Regulatory T cells overexpress a subset of Th2 gene transcripts. J Immunol. 2002. 168:1069–1079.

19. Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009. 30:626–635.

20. Graca L, Le Moine A, Cobbold SP, Waldmann H. Dominant transplantation tolerance. Opinion. Curr Opin Immunol. 2003. 15:499–506.
21. Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. "Infectious" transplantation tolerance. Science. 1993. 259:974–977.

22. Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000. 165:4783–4786.

23. Kendal AR, Waldmann H. Infectious tolerance: therapeutic potential. Curr Opin Immunol. 2010. 22:560–565.

24. Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002. 196:1335–1346.

25. Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001. 182:207–214.

26. Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010. 11:1093–1101.

27. Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+) CD25(-) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002. 168:5558–5565.

28. Feng G, Wood KJ, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008. 86:578–589.

29. Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004. 172:6003–6010.

30. Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007. 178:7018–7031.

31. Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005. 201:1037–1044.

32. Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, et al. In vivo tracking of 'color-coded' effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010. 16:718–722.

33. Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011. 208:2043–2053.

34. Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005. 115:3–9.

35. Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010. 185:2675–2679.

36. Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008. 181:8209–8213.

37. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002. 99:3493–3499.

38. Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012. 4:11–21.

39. Brennan TV, Tang Q, Liu FC, Hoang V, Bi M, Bluestone JA, et al. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. J Surg Res. 2011. 169:e69–e75.

40. Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007. 109:827–835.

41. Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010. 16:809–813.

42. Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011. 3:83ra42.

43. Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003. 112:1688–1696.

44. Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008. 118:3619–3628.

45. Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009. 30:458–469.

46. Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol. 2002. 3:1208–1213.

47. Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005. 174:6993–7005.

48. Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009. 21:281–285.

49. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009. 30:646–655.

50. Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010. 463:808–812.

51. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007. 5:e38.

52. Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009. 69:599–608.

53. Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011. 11:119–130.

54. Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999. 5:1303–1307.

55. Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant. 2008. 8:298–306.

56. Deeks ED, Keating GM. Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection. Drugs. 2009. 69:1483–1512.
57. Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006. 17:2844–2853.

58. Morelon E, Lefrançois N, Besson C, Prévautel J, Brunet M, Touraine JL, et al. Preferential increase in memory and regulatory subsets during T-lymphocyte immune reconstitution after Thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transpl Immunol. 2010. 23:53–58.

59. Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011. 187:2015–2022.

60. Quezada SA, Fuller B, Jarvinen LZ, Gonzalez M, Blazar BR, Rudensky AY, et al. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003. 102:1920–1926.

61. van Maurik A, Fazekas de St Groth B, Wood KJ, Jones ND. Dependency of direct pathway CD4+ T cells on CD40-CD154 costimulation is determined by nature and microenvironment of primary contact with alloantigen. J Immunol. 2004. 172:2163–2170.

62. Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008. 105:14527–14532.

63. Chen TC, Waldmann H, Fairchild PJ. Induction of dominant transplantation tolerance by an altered peptide ligand of the male antigen Dby. J Clin Invest. 2004. 113:1754–1762.

64. Sho M, Kishimoto K, Harada H, Livak M, Sanchez-Fueyo A, Yamada A, et al. Requirements for induction and maintenance of peripheral tolerance in stringent allograft models. Proc Natl Acad Sci U S A. 2005. 102:13230–13235.

65. Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999. 5:1298–1302.

67. Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008. 9:194–202.

68. Brandt C, Pavlovic V, Radbruch A, Worm M, Baumgrass R. Low-dose cyclosporine A therapy increases the regulatory T cell population in patients with atopic dermatitis. Allergy. 2009. 64:1588–1596.

69. Calvo-Turrubiartes M, Romano-Moreno S, García-Hernández M, Chevaile-Ramos JA, Layseca-Espinosa E, González-Amaro R, et al. Quantitative analysis of regulatory T cells in kidney graft recipients: a relationship with calcineurin inhibitor level. Transpl Immunol. 2009. 21:43–49.

70. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005. 105:4743–4748.

71. Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009. 30:832–844.

72. Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+T cells. Bone Marrow Transplant. 2007. 39:537–545.

73. Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007. 7:1722–1732.

74. Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007. 7:1819–1824.

75. Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009. 9:324–337.

76. Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008. 111:453–462.

78. Snanoudj R, Zuber J, Legendre C. Co-stimulation blockade as a new strategy in kidney transplantation: benefits and limits. Drugs. 2010. 70:2121–2131.

79. Wéclawiak H, Kamar N, Ould-Mohamed A, Cardeau-Desangles I, Rostaing L. Biological agents in kidney transplantation: belatacept is entering the field. Expert Opin Biol Ther. 2010. 10:1501–1508.

80. Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002. 196:401–406.
81. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003. 9:1144–1150.

82. Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127-T regulatory cells. Clin Immunol. 2009. 133:22–26.

83. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011. 117:1061–1070.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download