Abstract
The use of immunosuppressant's increases the risk of developing malignancies in renal allograft patients. One of the most important malignancies, Kaposi's sarcoma, can cause mortality and graft failure among renal allograft patients. We report the case of a 39-year-old male diagnosed with multiple visceral Kaposi's sarcoma 6 months after a second cadaveric renal allograft. The patient's renal function was markedly deteriorated at admission and required hemodialysis initially. Radiologic studies revealed Kaposi's sarcoma in multiple lymph nodes, liver, lung, and peritoneum. The excisional biopsy of an inguinal lymph node confirmed this diagnosis. After diagnosis, tacrolimus treatment was gradually decreased, and sirolimus treatment initiated. The patient did not receive any chemotherapy or radiotherapy. The Kaposi's sarcoma lesions decreased dramatically (both in size and number) 1 month after sirolimus treatment, and kidney graft function improved. This case thus shows successful sirolimus treatment of visceral Kaposi's sarcoma in a renal allograft patient.
Figures and Tables
 | Fig. 1Serum creatinine flow of the patient after second renal allograft from deceased donor. Abbreviations: DGF, delayed graft function; ACR, acute cellular rejection; BKVN, BK polyomavirus nephropathy; KS, Kaposi's sarcoma; ATG, antithymoglobulin; TAC, tacrolimus; MMF, mycophenolate mofetil; SRL, sirolimus. |
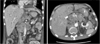 | Fig. 2Abdomino-pelvic computed tomography of the patient shows innumerable solid nodules in both lobes of liver, multiple lymphadenopathy involving periportal, celiac axis, and periaortic area. |
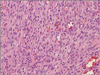 | Fig. 3Monomorphic spindle cells arranged in ill-defined fasicles, which are separated by slit-like vessels containing erythrocytes (HE stain, ×200). |
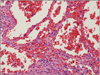 | Fig. 4Some areas show ectatic vascular spaces, lined by atypical endothelial cells (HE stain, ×200). |
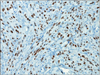 | Fig. 5Human herpesvirus 8 (HHV-8) immunohistochemical staining displays nuclear immunoreactivity in the endothelium and spindled component (HHV-8 stain, ×200). |
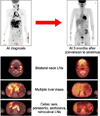 | Fig. 6Remission of Kaposi's sarcoma on positron emission tomography-computed tomography. The right figure shows markedly decreased degree of fludeoxyglucose uptakes in bilateral neck lymph nodes (LNs), liver mass, intra-abdominal lymph nodes with residual uptakes in the right mediastinum, left neck and axilla lymph nodes. |
 | Fig. 7Clinical course of the patient. Abbreviations: HD, hemodialysis; SRL, sirolimus; PET-CT, positron emission tomography-computed tomography. |
Table 1
Demographics and clinical characteristics of post-renal transplantation Kasposi's sarcoma cases since 1995

Abbreviations: KTx, kidney transplantation; KS, Kaposi's sarcoma; CNI, calcineurin inhibiotor; CsA, cyclosporine A; PR, partial response; LNs, lymph nodes; IS, immunosuppressant; CR, complete response; Tac, tacrolimus; IFN, interferone.
aTime form kidney transplantation to diagnosis of Kaposi's sarcoma (months).
References
1. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004. 4:905–913.

2. Andrés A. Cancer incidence after immunosuppressive treatment following kidney transplantation. Crit Rev Oncol Hematol. 2005. 56:71–85.

3. Hosseini-Moghaddam SM, Soleimanirahbar A, Mazzulli T, Rotstein C, Husain S. Post renal transplantation Kaposi's sarcoma: a review of its epidemiology, pathogenesis, diagnosis, clinical aspects, and therapy. Transpl Infect Dis. 2012. 14:338–345.

5. Duman S, Töz H, Aşçi G, Alper S, Ozkahya M, Unal I, et al. Successful treatment of post-transplant Kaposi's sarcoma by reduction of immunosuppression. Nephrol Dial Transplant. 2002. 17:892–896.

6. Moosa MR. Kaposi's sarcoma in kidney transplant recipients: a 23-year experience. QJM. 2005. 98:205–214.

7. Montagnino G, Bencini PL, Tarantino A, Caputo R, Ponticelli C. Clinical features and course of Kaposi's sarcoma in kidney transplant patients: report of 13 cases. Am J Nephrol. 1994. 14:121–126.

8. Einollahi B, Lessan-Pezeshki M, Nourbala MH, Simforoosh N, Pourfarziani V, Nemati E, et al. Kaposi's sarcoma following living donor kidney transplantation: review of 7,939 recipients. Int Urol Nephrol. 2009. 41:679–685.

9. Moray G, Başaran O, Yağmurdur MC, Emiroğlu R, Bilgin N, Haberal M. Immunosuppressive therapy and Kaposi's sarcoma after kidney transplantation. Transplant Proc. 2004. 36:168–170.

10. Charfi S, Krichen-Makni S, Yaich S, Makni H, Khabir A, Amouri A, et al. Successful treatment of post-renal transplant gastric and pulmonary Kaposi's sarcoma with conversion to rapamycin treatment. Saudi J Kidney Dis Transpl. 2007. 18:617–620.
11. Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005. 352:1317–1323.

12. Herman M, Weinstein T, Korzets A, Chagnac A, Ori Y, Zevin D, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001. 137:14–20.

13. Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999. 397:530–534.

14. Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003. 35:3 Suppl. 7S–14S.

15. Khokhar NZ, Altman JK, Platanias LC. Emerging roles for mammalian target of rapamycin inhibitors in the treatment of solid tumors and hematological malignancies. Curr Opin Oncol. 2011. 23:578–586.

17. Gutiérrez-Dalmau A, Sánchez-Fructuoso A, Sanz-Guajardo A, Mazuecos A, Franco A, Rial MC, et al. Efficacy of conversion to sirolimus in posttransplantation Kaposi's sarcoma. Transplant Proc. 2005. 37:3836–3838.

18. Mohsin N, Budruddin M, Pakkyara A, Darweesh A, Nayyer M, Amitabh J, et al. Complete regression of visceral Kaposi's sarcoma after conversion to sirolimus. Exp Clin Transplant. 2005. 3:366–369.
19. Gheith O, Bakr A, Wafa E, Fouda A, El Agroudy A, Refaie A, et al. Sirolimus for visceral and cutaneous Kaposi's sarcoma in a renal-transplant recipient. Clin Exp Nephrol. 2007. 11:251–254.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download