Abstract
Background
A management protocol for hepatitis C virus (HCV) after liver transplantation (LT) has not been established in Korea. We therefore investigated HCV transplant protocols and post-transplant results from liver transplant centers in Korea.
Methods
The HCV protocol and medical data of individual cases from eight major liver transplant centers were compiled and analyzed.
Results
A post-transplant protocol biopsy was performed in only three centers. In these centers, HCV treatment was considered when pathological abnormalities were confirmed on the protocol biopsy (irrespective of liver function). In the other five centers, biopsies were performed when biochemical parameters were aggravated. Only two out of the eight centers performed preemptive or prophylactic therapy. A total of 5,663 adult LTs were performed between 2000 and 2010. HCV-related liver disease was responsible for 277 LTs (4.9%). Pre-transplant data were not available in many patients, including HCV genotype and serum HCV RNA level. Tacrolimus was more frequently used for initial maintenance immunosuppression than cyclosporine A (61.7% vs. 36.8%). Post-transplant HCV treatment was performed in 135 patients (48.7%). Sixty-seven recipients (24.2%) died during follow-up after LT and 11 HCV-related graft loss (4.0%) developed. The cumulative patient survival rate was 74.7% at 5 years and 67.9% at 10 years after LT.
Conclusions
The HCV management protocol after LT varied markedly between the eight Korean transplant centers and a standard protocol did not exist. A nationwide multicenter study is required to investigate the most effective treatment for HCV after LT, with the goal of establishing the most effective standard protocol.
In addition to alcoholic liver disease, hepatitis C virus (HCV)-related liver disease is a major indication for liver transplantation (LT) worldwide. It is second most common indication for LT in the United States and northern Europe, and the primary indication in southern Europe countries with high HCV prevalence, such as Italy and Spain(1).
LT has been accepted and is widely-used as the only curative treatment modality for HCV-related cirrhosis and hepatocellular carcinoma (HCC). HCV, however, universally recurs after LT(2,3) and its recurrence frequently proceeds to graft failure and mortality(4,5). Recurrence of HCV infection is immediate in recipients who are serum HCV RNA-positive at the time of LT(6). Although <20% of patients transplanted for HCV may have no significant lesions 5 years after LT, most recipients display progressive chronic hepatitis, which is clearly more aggressive than in immunocompetent subjects, and leads to cirrhosis in 10~50% of recipients at 5 years post-LT(7-9). Moreover, in <5% of patients, HCV recurrence is very severe and rapidly progressive, corresponding to a fibrosing cholestatic hepatitis, and resulting in liver failure in a few weeks or months(10). Therefore, the transplant results of HCV are inferior to those of other indications(1).
The improvement of post-transplant outcomes for HCV is still a challenging task in the liver transplant field. Many risk factors associated with HCV recurrence and poor survival have been reported and include HCV genotype 1, high viral load, female gender, long ischemic time, blood transfusion, steatosis of the graft, old age of the donor age, and use of steroids or antilymphocytes(1). In addition, sustained virological response (SVR) by antiviral treatment improves post- transplant survival(10). Based on these reports and individual experiences, worldwide major transplant centers have independently developed and used HCV management protocols with the goal of improving the survival of recipients with HCV.
Korea is an endemic hepatitis B virus (HBV) region. Here, HBV accounts for more than 75% of all causes of adult LT, while HCV is responsible for <10% of adult LT. A few established post-transplant management methods for HBV are generally accepted in Korea and the transplant results are similar among transplant centers. However, few studies have been performed on patients transplanted for HCV-related liver disease and there is no general consensus among the transplant centers about the results and management protocol of LT for HCV.
This study was performed to investigate the transplant experiences for HCV of Korea, with the aim of providing preliminary data for future consensus on the management of HCV.
The medical data of patients who underwent LT for HCV-related liver disease between 2000 and 2010 was collected from eight major liver transplant centers of Korea: Seoul National University Hospital (SNUH), National Cancer Center (NCC), Asan Medical Center (AMC), Samsung Medical Center, Yonsei Medical Center, Catholic Medical Center (CMC), Ajou University Hospital, and Daegu Catholic Medical Center (DCMC).
The collected variables were the annual number of adult (≥18 years of age) LT, management protocol, and individual patient data. The latter included sex, age, HCV genotype, pre-transplant viral load, pre- and post-transplant HCV treatment and its response, immunosuppression, rejection, survival, and cause of death.
Protocol biopsy was defined as scheduled histopathological examination, which was performed irrespective of clinical or laboratory deterioration.
Post-transplant anti-HCV therapy was divided according to preemptive, prophylactic, and therapeutic intent. Preemptive or prophylactic treatment was early post-transplant therapy performed before HCV hepatitis or cirrhosis was histopathologically confirmed. Preemptive treatment was defined as anti-viral therapy that was started when the serum HCV viral load increased. Prophylactic treatment was defined as universal therapy routinely performed in all recipients with HCV. Post-transplant anti-HCV treatment performed after histopathological confirmation of HCV hepatitis or cirrhosis was considered as therapeutic treatment.
Eight centers responded with information of their institution's HCV management protocol. No center had a definite pre-transplant management principle for HCV. Therefore, although anti-viral therapy was performed in some patients before LT, it was not regular. In addition, some important laboratory tests such as HCV genotype and viral load were not checked preoperatively in many patients.
Post-transplant protocol biopsy was performed only in three centers (SNUH, NCC, and CMC). Regular biopsies were performed in these centers irrespective of liver function abnormality or HCV viral load. Protocol biopsy was performed every year after LT and additionally performed at 3 and 6 months post-LT in SNUH and NCC. Anti-viral therapy was considered when HCV hepatitis or fibrosis was confirmed on histopathological examination, irrespective of liver function abnormality. Conversely, a scheduled protocol biopsy was not routinely performed in the other centers. Pathological examination was tried only when biochemical parameters were aggravated.
Preemptive treatment for HCV was performed only in DCMC. In this center, anti-HCV therapy was started when a serum viral load increase was observed. The referred level of serum HCV RNA associated with anti-viral therapy, however, was not known. AMC has implemented universal prophylaxis since 2008, which involved ribavirin (RIB) and conventional interferon (IFN) with a low dosage at 3~4 weeks post-LT in all recipients with HCV, gradually increasing the dosage and then finally changed conventional INF to pegylated IFN (Peg-INF). In the other six centers, anti-HCV treatment was performed as a therapeutic manner. It was used only when HCV hepatitis was confirmed biochemically and pathologically.
Individual patient data was collected from seven centers except DCMC. A total of 5,663 adult LT were performed in these centers between 2000 and 2010. Among them, HCV-related liver disease was responsible for 277 LTs (4.9%). The annual proportion of LT for HCV fluctuated during the study period, but largely showed a gradually increasing pattern (Fig. 1). One hundred eighty-five patients (66.8%) were male and 92 (33.2%) were female. Mean age of the patients was 55.7±0.49 years at the time of LT. One hundred forty-eight patients (53.4%) had HCC preoperatively. Thirty-nine patients (14.1%) had co-infection of HBV and HCV and one (0.4%) had human immunodeficiency virus infection preoperatively (Table 1).
Pre-transplant data were not available in many patients, including HCV genotype and serum HCV RNA level. Pre-transplant serum HCV RNA level could not analyzed because HCV RNA was not preoperatively tested or was checked qualitatively in some patients. In addition, the analysis method and measurement unit differed, even in patients for whom quantitative data were available. The most frequent HCV genotype was 1b (45.8%), followed by 2a (26.4%) (Fig. 2). HCV genotype, however, was not available in 64 patients (23.1%).
Anti-HCV treatment was tried in 52 patients (18.8%) before LT. The combination of RIB and Peg-IFN was most frequently used (Fig. 3). Treatment was usually maintained for 6~24 months in most patients, excluding those who underwent LT during antiviral therapy. Two patients received the combination therapy of 48 months and one received long-term RIB monotherapy following a 6-month-combination regimen.
Induction therapy was tried in 69.7% of patients, with basiliximab used in all cases. Tacrolimus (TAC) was more frequently used for initial maintenance therapy (61.7%) than cyclosporine A (CSA) (36.8%). Mycophenolate mofetil was added to calcineurin inhibitors (CNIs) in 67.9% of patients (Fig. 4). Corticosteroid was used in most patients during early post-LT period. It was tapered off and discontinued by 6 months post-LT in 66.3% of 240 patients who survived for more than 6 months after LT and by 3 months in 30.4%. Biopsy-proven acute rejection with a rejection activity index ≥4 developed in 52 patients (18.8%) and steroid pulse therapy was tried in 30.8% of these patients.
CNIs were changed 44 times in 40 patients during the follow-up. The change from CSA to TAC was done 25 times. The most common cause of the change was rescue for rejection (76.0%). On the other hand, the change from TAC to CSA was done 17 times and, in three quarters of cases, was because of HCV hepatitis or TAC-related side-effects (Table 2).
Median follow-up duration of patients was 28.0 (0.1~128.6) months after LT.
Protocol liver biopsy was performed in 72 patients (26.0%) and first protocol biopsy was frequently carried out within 3 months post-LT (63.9%). Post-transplant anti-HCV therapy was performed in 135 patients (48.7%). It was tried as prophylactic intent in 59 patients (21.3%) and performed for hepatitis treatment on 76 patients (27.4%). Because individual data from DCMC were not collected, the number of patients with preemptive therapy was not surveyed. Median duration of anti-HCV treatment was 12.0 (1.0~96.0) months. The most common regimen, combination of RIB and Peg-INF was more frequently used (77.0%) than in pre-transplant treatment, especially when used as prophylactic intent (91.5%) (Fig. 3). After anti-HCV treatment, end-of-time virological response (ETVR) and SVR was achieved in 79 patients (58.5%) and 59 patients (43.7%), respectively. However, 29 patients (21.5%) did not show the virological response. The response was not assessable in 27 patients (20.0%) because of short treatment duration or missing data. Therefore, actual ETVR and SVR might be higher with proper treatment and follow-up.
Sixty-five recipients (23.5%) died during follow-up after LT. The most common cause of death was infectious diseases such as pneumonia and septic shock, followed by HCC recurrence (Table 3). There were seven mortalities related to recurrent HCV and six patients underwent re-transplantation due to HCV-related graft failure. Thus, of all cases, 13 HCV-related graft losses (4.7%) developed after LT. Cumulative patient survival rate was 74.7% at 5 years and 67.9% at 10 years after LT (Fig. 5). Graft survival and cumulative HCV recurrence rate were not analyzed because the data of HCV recurrence and graft failure was not fully investigated in this study.
HCV genotype is a well-known risk factor associated with post-transplant survival(1). Patients with higher pre-transplant HCV RNA titers experience greater mortality and graft loss rate than patients with lower titers(11). In Korea, pre-transplant management of HCV was not strict. HCV genotype and viral load were not checked preoperatively in many cases. These laboratory tests are strongly recommended before LT and pre-transplant antiviral therapy should be considered when HCV RNA titers are high. Antiviral treatment, however, is poorly tolerated in patients with severe liver disease due to high incidence of serious adverse events(12). Thus, the International Liver Transplant Society consensus panel concluded that antiviral treatment should be limited to cirrhotic patients with Child-Turcotte-Pugh (CTP) score ≤7 or Model of End Staged Liver Disease (MELD) score <18, and be contraindicated when the CTP is >11 or MELD score is >25(13).
Although TAC (61.7%) was more frequently used in LT for HCV than CSA (31.8%) in the surveyed centers in this study, it seems that the use of CSA is more common in recipients with HCV than in recipients with other disease. This may result from recent in vitro findings that CSA could have a beneficial effect on patients with HCV. Ishii et al.(14) reported that CSA significantly inhibits HCV replication in the replicon model. Consequently, there is hope that CSA could reduce the severity of HCV recurrence(15). However, the in vitro effects of CSA on HCV have not yet been realized in clinical settings. In a meta-analysis, no difference was found in patient and graft survival for liver transplant recipients receiving either CSA or TAC(16). In all but one of these studies, the outcomes with respect to HCV were measured 1-year after LT, that is, before the natural history of recurrent HCV was allowed to develop. Therefore, continued follow-up analyses of these groups may have yielded different results(15). In fact, the only study evaluating outcomes after 1 year showed that the long-term outcomes of HCV recipients treated with CSA were significantly worse than those of HCV recipients treated with TAC(17). Another meta-analysis of studies comparing CSA and TAC also found a patient and graft survival benefit associated with TAC as maintenance immunosuppression(18). Hence, based on these reports, TAC is recommended as an initial maintenance CNI rather than CSA.
However, there is emerging evidence that CSA may have an impact on HCV biology that requires concomitant administration of IFN(13). In most recent studies that examined the impact of CNIs with respect to the responsiveness to IFN therapy after LT, CSA has been associated with a higher SVR rate in comparison with TAC(19-22). In multivariate analysis, CSA was independently associated with a significantly higher SVR rate(20,21). This may result from the antiviral effect of CSA, which inhibits the binding NS5B to cyclophilin B(13). Thus, the change of CNI to CSA during IFN-based antiviral therapy is persuasive.
Early prophylactic HCV treatment has been recently tried to improve the prognosis of recipients with HCV. Some randomized, controlled studies about universal prophylactic therapy have been performed or are underway. The efficacy of early HCV prophylaxis, however, has not been yet established(13,23). Treatment in the early post-operative period seems to be acceptably tolerable, but yields a very poor efficacy(13). Preemptive or prophylactic therapy is not frequently performed in Korea and there no study data are available. Therefore, Korean studies about early post-transplant HCV prophylaxis are required to establish practical guidelines for recipients with HCV.
Presently, post-transplant HCV treatment regimen was Peg-IFN and RIB in most cases. Recent studies utilizing these drugs have reported more favorable outcomes. In the aggregate, using standard dosing of Peg-IFN and RIB, approximately 50% ETR and 30~35% SVR can be expected with a 48-week-duration therapy for patients with genotype 1. Thus, the efficacy of Peg-IFN and RIB is approximately one third, which is less than in non-transplant settings(13).
According to previous reports, survival of patients with HCV can reach 61~75% and 68% at 5 and 10 years after transplantation(24-26). The results of this study were not superior to results from studies from Western countries and no racial influence was apparent. However, as expected, the post-transplant results of HCV were inferior to other indications, especially HBV. Under the proper prophylaxis, 10-year-survival reaches approximately 80% in HBV(27). This inferiority of HCV was presumed to result from the difficulty of prophylaxis for recurrent disease. The outcome of transplantation for HBV had been worse than HCV before effective antiviral agents and HBV immunoglobulin were developed and clinically used. However, the drugs for HCV are not only less effective but also less tolerable than HBV treatment agents. Therefore, to improve post-transplant HCV treatment agents, basic and clinical studies are expected to be continuously tried.
HCV has not been attractive subject for study in Korea because its prevalence is much lower than HBV. While HBV management methods and transplant results are similar among transplant centers, the HCV management protocol varied markedly between transplant centers and the outcomes of recipients with HCV are not well-known. However, the frequency of LT for HCV is expected to increase and its prognosis is still a challenging issue. Therefore, a nationwide multicenter study is required to investigate the transplant results of HCV and to establish the most effective standard protocol.
Figures and Tables
 | Fig. 1Annual proportion of liver transplantation for hepatitis C virus (HCV). The annual proportion of liver transplantation for HCV fluctuated during the study period, but largely showed gradual increasing pattern. |
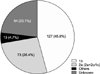 | Fig. 2Hepatitis C virus (HCV) genotypes. The most frequent HCV genotype was 1b (45.8%), followed by 2a (26.4%). |
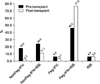 | Fig. 3Hepatitis C virus (HCV) treatment regimen. The most common HCV treatment regimen was the combination of pegylated interferon (Peg-IFN) and ribavirin (RIB). It was used in about half the cases before liver transplantation. However, it was far more frequently used in post-transplant treatment. |
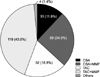 | Fig. 4The initial regimen of maintenance immunosuppressive therapy. Tacrolimus (TAC) was more frequently used for initial maintenance therapy than cyclosporine A (CSA). Mycophenolate mofetil (MMF) was added to calineurin inhibitors in patients of 67.9%. Corticosteroid was excluded in diagram because it was used in most patients. |
 | Fig. 5Cumulative patient survival of liver transplantation for hepatitis C virus. Cumulative patient survival rate was 74.7% at 5 years and 67.9% at 10 years after liver transplantation. |
References
1. Guillouche P, Féray C. Systematic review: anti-viral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011. 33:163–174.

2. Féray C, Samuel D, Thiers V, Gigou M, Pichon F, Bismuth A, et al. Reinfection of liver graft by hepatitis C virus after liver transplantation. J Clin Invest. 1992. 89:1361–1365.

3. Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992. 103:317–322.

4. Prieto M, Berenguer M, Rayón JM, Córdoba J, Argüello L, Carrasco D, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999. 29:250–256.

5. Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002. 8:10 Suppl 1. S14–S18.

6. Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996. 110:167–177.

7. Féray C, Gigou M, Samuel D, Paradis V, Wilber J, David MF, et al. The course of hepatitis C virus infection after liver transplantation. Hepatology. 1994. 20:1137–1143.

8. Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996. 334:815–820.

9. Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000. 32:673–684.

10. Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, et al. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007. 46:459–465.

11. Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998. 28:823–830.

12. Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005. 42:255–262.

13. Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009. 9:1707–1713.

14. Ishii N, Watashi K, Hishiki T, Goto K, Inoue D, Hijikata M, et al. Diverse effects of cyclosporine on hepatitis C virus strain replication. J Virol. 2006. 80:4510–4520.

15. Trotter JF. Hot-topic debate on hepatitis C virus: the type of immunosuppression matters. Liver Transpl. 2011. 17:Suppl 3. S20–S23.

16. Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007. 13:21–29.

17. Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998. 66:493–499.

18. McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006. 6:1578–1585.

19. Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007. 132:1746–1756.

20. Selzner N, Renner EL, Selzner M, Adeyi O, Kashfi A, Therapondos G, et al. Antiviral treatment of recurrent hepatitis C after liver transplantation: predictors of response and long-term outcome. Transplantation. 2009. 88:1214–1221.

21. Cescon M, Grazi GL, Cucchetti A, Vetrone G, Ravaioli M, Ercolani G, et al. Predictors of sustained virological response after antiviral treatment for hepatitis C recurrence following liver transplantation. Liver Transpl. 2009. 15:782–789.

22. ReViS-TC Study Group. Cyclosporine a-based immunosuppression reduces relapse rate after antiviral therapy in transplanted patients with hepatitis C virus infection: a large multicenter cohort study. Transplantation. 2011. 92:334–340.
23. Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005. 41:289–298.

24. Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002. 122:889–896.

25. Berenguer M, Prieto M, San Juan F, Rayón JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002. 36:202–210.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download