Abstract
Background
Compliance from kidney transplant recipients might improve with less frequent doses of immunosuppressant drugs. We describe the development of an extended-release formulation of tacrolimus that enables taking the drug just once a day, instead of the current twice a day tacrolimus formulation.
Methods
We performed a prospective, open-label, 1:1 randomized, and multicenter study. Patients received Prograf® (Astellas Inc.) twice a day for 1 month post-transplantation. The patients of the investigational group converted to a dose of Advagraf® (Astellas Inc.) given once a day. We evaluated the efficacy, safety, and patient satisfaction of both groups.
Results
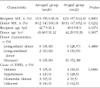
Within 5 months after conversion to Advagraf, the incidence of biopsy-confirmed acute rejection was 0%, while patient and graft survival was 100%. We could not find differences of the patients' estimated glomerular filtration rate (eGFR) between the Prograf and Advagraf treated groups 1~6 months post-transplantation. The safety profile and satisfaction profiles (immunosuppressant therapy barrier scale) were also equivalent between the treated groups.
Figures and Tables
References
2. First MR, Fitzsimmons WE. New drugs to improve transplant outcomes. Transplantation. 2004. 77:9 Suppl. S88–S92.

3. Regazzi MB, Alessiani M, Rinaldi M. New strategies in immunosuppression. Transplant Proc. 2005. 37:2675–2678.

4. Ponticelli C, Villa M, Cesana B, Montagnino G, Tarantino A. Risk factors for late kidney allograft failure. Kidney Int. 2002. 62:1848–1854.

5. Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002. 346:580–590.

6. Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005. 16:1839–1848.

7. Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004. 77:769–776.

8. Bunzel B, Laederach-Hofmann K. Solid organ transplantation: are there predictors for posttransplant noncompliance? A literature overview. Transplantation. 2000. 70:711–716.

9. Feinstein S, Keich R, Becker-Cohen R, Rinat C, Schwartz SB, Frishberg Y. Is noncompliance among adolescent renal transplant recipients inevitable? Pediatrics. 2005. 115:969–973.

10. Vlaminck H, Maes B, Evers G, Verbeke G, Lerut E, Van Damme B, et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004. 4:1509–1513.

11. De Geest S, Vanhaecke J. Methodological issues in transplant compliance research. Transplant Proc. 1999. 31(4A):81S–83S.

12. Chisholm MA, Lance CE, Williamson GM, Mulloy LL. Development and validation of an immunosuppressant therapy adherence barrier instrument. Nephrol Dial Transplant. 2005. 20:181–188.

13. Wente MN, Sauer P, Mehrabi A, Weitz J, Büchler MW, Schmidt J, et al. Review of the clinical experience with a modified release form of tacrolimus [FK506E (MR4)] in transplantation. Clin Transplant. 2006. 20:Suppl 17. 80–84.

14. Mourad M, Malaise J, Chaib Eddour D, De Meyer M, König J, Schepers R, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem. 2001. 47:88–94.

15. Kagaya H, Miura M, Satoh S, Inoue K, Saito M, Inoue T, et al. No pharmacokinetic interactions between mycophenolic acid and tacrolimus in renal transplant recipients. J Clin Pharm Ther. 2008. 33:193–201.

16. Neumayer HH. Introducing everolimus (Certican) in organ transplantation: an overview of preclinical and early clinical developments. Transplantation. 2005. 79:9 Suppl. S72–S75.

17. Rintala JM, Savikko J, Rintala SE, von Willebrand E. FK778 ameliorates post-transplant expression of fibrogenic growth factors and development of chronic rejection changes in rat kidney allografts. Nephrol Dial Transplant. 2008. 23:3446–3455.

18. Baraldo M, Furlanut M. Chronopharmacokinetics of ciclosporin and tacrolimus. Clin Pharmacokinet. 2006. 45:775–788.

19. Bekersky I, Dressler D, Mekki Q. Effect of time of meal consumption on bioavailability of a single oral 5 mg tacrolimus dose. J Clin Pharmacol. 2001. 41:289–297.

20. Bekersky I, Dressler D, Mekki QA. Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J Clin Pharmacol. 2001. 41:176–182.

21. Park SI, Felipe CR, Pinheiro-Machado PG, Garcia R, Tedesco-Silva H Jr, Medina-Pestana JO. Circadian and time-dependent variability in tacrolimus pharmacokinetics. Fundam Clin Pharmacol. 2007. 21:191–197.

22. Choi JH, Lee YJ, Jang SB, Lee JE, Kim KH, Park K. Influence of the CYP3A5 and MDR1 genetic polymorphisms on the pharmacokinetics of tacrolimus in healthy Korean subjects. Br J Clin Pharmacol. 2007. 64:185–191.

23. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004. 43:623–653.

24. Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002. 72:660–669.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download