Abstract
Background
Since the time various strategies have been introduced to overcome the ABO-blood barrier including local infusion therapy (LIT), plasmapheresis and rituximab, the graft and patient survival outcome of ABO-incompatible (ABOi) adult living donor liver transplantation (ALDLT) has remarkably improved. But, the need for LIT under rituximab prophylaxis should be reevaluated because of high incidence of the LIT-related complications. The aim of this study was to verify the safety and efficacy of the protocol without local infusion therapy in ABOi ALDLT.
Methods
From November 2008 to December 2010, 43 cases of ABO-incompatible adult living donor liver transplantation were performed. In all cases, the spleen was preserved. From the 1st to 20th case, LIT was employed (group I, n=20). From the 21th case onwards, LIT was eliminated from the protocol (group II, n=23). The 3-month and 1-year patient and graft survival rates were compared between the two groups. The clinical parameters including recipient, donor and graft-related factors were also compared. The graft function was assessed in each group based on the serial changes in serum AST/ALT, total bilirubin and prothrombin time.
Results
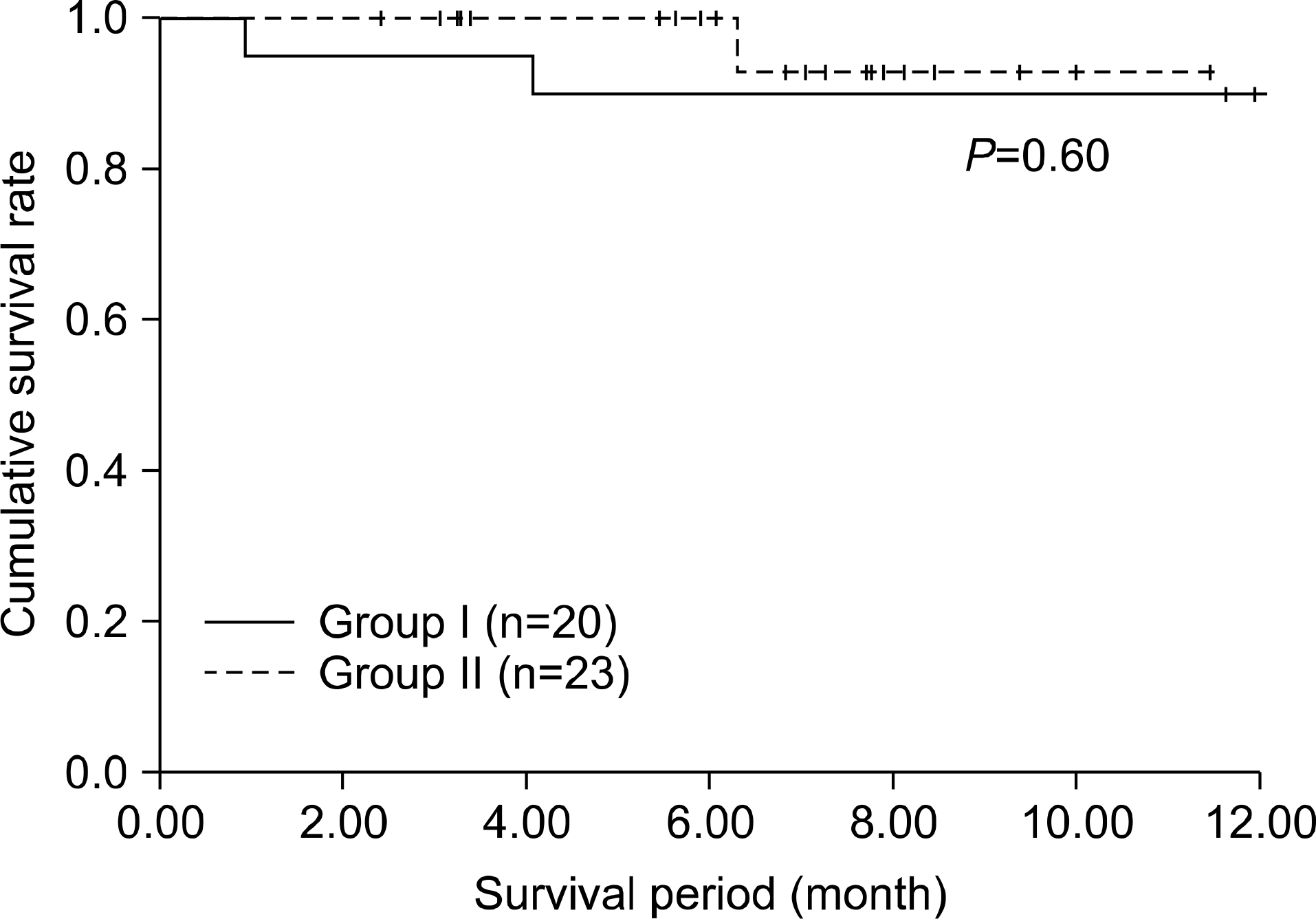
There was 1 case of in-hospital mortality (2.3%) among the 43 cases. Overall 3-month and 1-year patient and graft survival rate was 97.7% and 92.1% during a mean period of 11.4 ± 0.4 (0.9∼28.9) months. There was no significant difference in the 3-month and 1-year patient and graft survival rates (95.0 vs. 100% and 90.0 vs. 92.9%, P=0.60) between groups. LIT-related complications occurred in 4 patients (20.0%). One case of antibody-mediated rejection occurred in group II. Both groups showed no difference in graft function at postoperative 3rd month.
Go to : 
References
1). Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990; 336:519–23.

2). Reding R, Veyckemans F, de Ville de Goyet J, de Hemptinne B, Carlier M, Van Obbergh L, et al. ABO-incompatible orthotopic liver allografting in urgent indications. Surg Gynecol Obstet. 1992; 174:59–64.
3). Lo CM, Shaked A, Busuttil RW. Risk factors for liver transplantation across the ABO barrier. Transplantation. 1994; 58:543–7.

4). Egawa H, Ohdan H, Haga H, Tsuruyama T, Oike F, Uemoto S, et al. Current status of liver transplantation across ABO blood-type barrier. J Hepatobiliary Pancreat Surg. 2008; 15:131–8.

5). Kawagishi N, Satomi S. ABO-incompatible living donor liver transplantation: new insights into clinical relevance. Transplantation. 2008; 85:1523–5.

6). Tanabe M, Shimazu M, Wakabayashi G, Hoshino K, Kawachi S, Kadomura T, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002; 73:1959–61.
7). Yoshizawa A, Sakamoto S, Ogawa K, Kasahara M, Uryuhara K, Oike F, et al. New protocol of immunosuppression for liver transplantation across ABO barrier: the use of Rituximab, hepatic arterial infusion, and preservation of spleen. Transplant Proc. 2005; 37:1718–9.

8). Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008; 47:143–52.

9). Hanto DW, Fecteau AH, Alonso MH, Valente JF, Whiting JF. ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: evidence for accommodation. Liver Transpl. 2003; 9:22–30.

10). Troisi R, Noens L, Montalti R, Ricciardi S, Philippe J, Praet M, et al. ABO-mismatch adult living donor liver transplantation using antigen-specific immunoadsorption and quadruple immunosuppression without splenectomy. Liver Transpl. 2006; 12:1412–7.

11). Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Harada N, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009; 88:303–7.

12). Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988; 132:489–502.
13). Farges O, Kalil AN, Samuel D, Saliba F, Arulnaden JL, Debat P, et al. The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplantation. 1995; 59:1124–33.
14). Chui AK, Ling J, McCaughan GW, Painter D, Shun A, Dorney SF, et al. ABO blood group incompatibility in liver transplantation: a single-centre experience. Aust N Z J Surg. 1997; 67:275–8.

15). Egawa H, Oike F, Buhler L, Shapiro AM, Minamiguchi S, Haga H, et al. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004; 77:403–11.

16). Ohdan H, Zhou W, Tanaka Y, Irei T, Fuchimoto Y, Egawa H, et al. Evidence of immune tolerance to blood group antigens in a case of ABO-incompatible pediatric liver transplantation. Am J Transplant. 2007; 7:2190–4.

17). Tanaka Y, Ohdan H, Zhou W, Onoe T, Hara H, Tokita D, et al. Enzyme-linked immunospot assay for detecting cells secreting antibodies against human blood group A epitopes. Transplant Proc. 2003; 35:555–6.

18). Pescovitz MD. B cells: a rational target in alloantibody-mediated solid organ transplantation rejection. Clin Transplant. 2006; 20:48–54.

19). Irei T, Ohdan H, Zhou W, Ishiyama K, Tanaka Y, Ide K, et al. The persistent elimination of B cells responding to blood group A carbohydrates by synthetic group A carbohydrates and B-1 cell differentiation blockade: novel concept in preventing antibody mediated rejection in ABO-incompatible transplantation. Blood. 2007; 110:4567–75.
20). Takahashi K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody mediated rejection due to ABO-bloodgroup-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007; 11:128–41.
21). Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, et al. Successful use of an-ti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living related liver transplantation. Transplantation. 2005; 79:12–6.
22). Pierson RN 3rd, Loyd JE, Goodwin A, Majors D, Dummer JS, Mohacsi P, et al. Successful management of an ABO-mismatched lung allograft using antigen-specific immunoadsorption, complement inhibition, and immunomodulatory therapy. Transplantation. 2002; 74:79–84.
23). Shimazu M, Kitajima M. Living donor liver transplantation with special reference to ABO-incompatible grafts and small-for-size grafts. World J Surg. 2004; 28:2–7.

24). Kim BW, Park YK, Kim YB, Wang HJ, Kim MW. Effects and problems of adult ABO-incompatible living donor liver transplantation using protocol of plasma exchange, intraarterial infusion therapy, and anti-CD20 monoclonal antibody without splenectomy: case reports of initial experiences and results in Korea. Transplant Proc. 2008; 40:3772–7.

25). Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011; 41:317–22.

26). Neron S, Lemieux R. CD5+ B cell-dependent regulation of the murine T-cell independent immune response against the human blood group A antigen. Immunol Invest. 1997; 26:631–47.
27). Neron S, Lemieux R. Type 2 T-cell-independent murine immune response to the human ABO blood group antigens. Vox Sang. 1994; 67:68–74.
28). Usui M, Isaji S, Mizuno S, Sakurai H, Uemoto S. Experiences and problems preoperative anti-CD20 monoclonal antibody infusion therapy with splenectomy and plasma exchange for ABO-incompatible living-donor liver transplantation. Clin Transplant. 2007; 21:24–31.

Go to : 
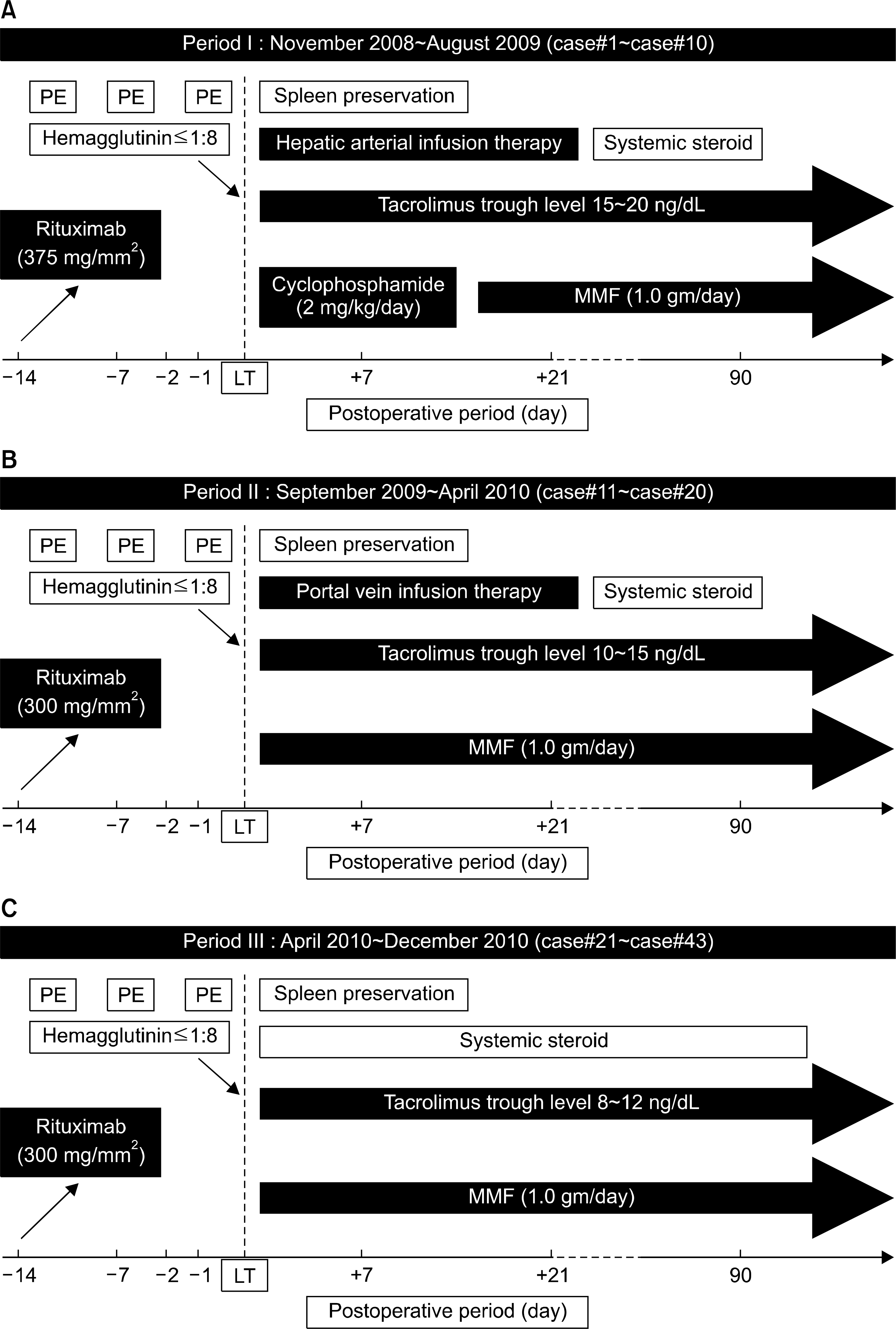 | Fig. 1.The change of immunosuppression protocol for ABOi LDLT. (A) From Nov 2008 to Aug 2009, hepatic arterial infusion, cyclophosphamide (2 mg/kg/day), high level of tacrolimus trough level (15∼20 ng/dl) and 375 mg/BSA mm2 rituximab have been employed for first 10 patients (case #1∼ case #10). (B) From Sep 2009 to Apr 2010, portal vein infusion, lower level of tacrolimus trough level (10∼15 ng/dl) and reduced dosage of rituximab (300 mg/BSA mm2) have been used for 10 patients (case #11∼case #20). Cyclophosphamide was eliminated from protocol due to frequent side effect. (C) Since Apr 2010, tacrolimus trough level has been reduced further more (8∼12 ng/dl). And local infusion has been abandoned for remaining 23 patients (case #21∼case #43). |
 | Fig. 3.Hepatic artery injuries by the insertion of catheter for local infusion therapy. (A) Hepatic artery injury (white arrow) was detected by doppler ultrasonography and hepatic arteriography on postoperative 1st day. (B) Hepatic artery injury (white arrow) was detected during the procedure of hepatic arterial catheter insertion under fluoroscopy. Hepatic artery injury in both cases was successfully corrected by surgical revision with right gastroepiploic artery. |
Table 1.
ABO blood type relation between recipient and donor
Table 2.
Forty three complications occurred in 23 patients after ABOi living donor liver transplantation
Table 3.
Comparison of demographic and clinical data of recipient and donor between group I and II
| Variable | Group I (n=20) | Group II (n=23) | P value |
|---|---|---|---|
| Recipient age | 49.8±6.5(38∼62) | 46.9±9.5 (25∼68) | 0.95 |
| Recipient sex (M/F) | 16 (80.0%)/4 | 15 (65.2%)/8 | 0.28 |
| Original disease | HBV 17(85.0%) | HBV 20 (87.0%) | 0.73 |
| HCV 1 (5.0%) | HCV 0 | ||
| Alcoholic LC 1 (5.0%) | Alcoholic LC 1 (4.3%) | ||
| Wilson’ s disease 1 (5.0%) | Wilson’ s disease 1 (4.3%) | ||
| Cryptogenic LC 0 | Cryptogenic LC 1 (4.3%) | ||
| Combined HCC (Y/N) | 5 (25.0%)/15 | 12 (52.2%)/11 | 0.07 |
| Recipient ABO type | A 4 (20.0%) | A 6 (26.1%) | 0.20 |
| B 6 (30.0%) | B 2 (8.7%) | ||
| O 10 (50.0%) | O 15 (65.2%) | ||
| Recipient-donor | A ← B 2 (10.0%) | A ← B 4 (17.4%) | 0.32 |
| ABO match | ← AB 2 (10.0%) | ← AB 2 (8.7%) | |
| B ← A 2 (10.0%) | B ← A 0 | ||
| ← AB 4 (20.0%) | ← AB 2 (8.7%) | ||
| O ← A 7 (35.0%) | O ← A 8 (34.8%) | ||
| ← B 2 (10.0%) | ← B 7 (30.4%) | ||
| ← AB 1 (5.0%) | ← AB 0 | ||
| MELD score | 15.4±5.3 (8∼25) | 13.5±6.0 (6∼29) | 0.28 |
| CTP score | 8.3±6.5 (6∼11) | 7.3 ± 1.8 (6∼10) | 0.08 |
| Graft type | MRL 14 (70.0%) | MRL 20 (87.0%) | 0.25 |
| ERL 0 | ERL 1 (4.3%) | ||
| Dual 5 (25.0%) | Dual 2 (8.7%) | ||
| LL+S1 1 (5.0%) | LL+S1 0 | ||
| GRWR (%) | 1.1±0.3 (0.8∼1.8) | 1.2±0.3 (0.7∼2.1) | 0.39 |
| Graft fatty change (%) | 5.9±8.9 (0∼10) | 2.9±4.5 (0∼20) | 0.14 |
| Donor age (years) | 29.1±9.7 (15‡∼53) | 30.3±10.2 (16∼55) | 0.67 |
| Donor sex (M/F) | 19 (76.0%)/6 | 16 (64.0%)/9 | 0.36 |
| Donor BMI | 23.5±5.4 (20.3∼32.4) | 23.4±2.2 (19.1∼28.3) | 0.95 |
| Operation time (minute) | 917.9±182.3 (685∼1101) | 806.2±100.3 (605∼1014) | 0.02 |
| Transfusion of P-RBC* (unit) | 13.0±17.7 (3∼107) | 6.3±7.5 (2∼50) | 0.02 |
| Total ischemic time (minute) | 143.5±54.9 (92∼189) | 125.0±25.8 (83∼201) | 0.18 |
| Length of hospital stay†(day) | 46.3±21.3 (26∼99) | 31.9±12.5 (17∼76) | 0.01 |
| Follow-up period (month) | 17.7±7.5 (0.9∼28.9) | 6.7±2.5 (3.0∼11.7) | 0.00 |
Table 4.
Incidence (number of patient) of postoperative complication in each group
Table 5.
Comparison of graft function by serum AST/ALT, total bilirubin and prothrombin time between group I and II
| Variable | Group I (n=19*) | Group II (n=23) | P value |
|---|---|---|---|
| Serum AST (IU/L) | |||
| Preoperative | 36.0±14.9 (17∼75) | 35.6±12.3 (17∼64) | 0.95 |
| Postoperative 7 th day | 51.3±31.8 (21∼164) | 50.4±27.1 (16∼138) | 0.93 |
| Postoperative 1 st month | 111.2±348.8 (15∼1550†) | 34.9±24.4 (13∼119) | 0.35 |
| Postoperative 3 rd month | 25.6±10.4 (11∼47)† | 24.5±10.4 (14∼57) | 0.75 |
| Peak | 556.5±912.3 (103∼4086†) | 306.2±180.5 (66∼708) | 0.24 |
| Required time for normalization (day) | 11.7±14.2 (3∼67) | 12.3±13.6 (1∼54) | 0.88 |
| Serum ALT (IU/L) | |||
| Preoperative | 22.9±10.5 (9∼48) | 23.0±8.6 (12∼40) | 0.99 |
| Postoperative 7 th day | 93.0±51.3 (23∼210) | 97.8±44.6 (30∼167) | 0.75 |
| Postoperative 1 st month | 99.5±159.8 (10∼713†) | 81.5±72.3 (5∼309) | 0.65 |
| Postoperative 3 rd month | 26.4±23.7 (6∼105) | 34.5±28.3 (8∼125) | 0.32 |
| Peak | 378.1±111.9 (101∼1363†) | 378.0±172.4 (64∼676) | 0.31 |
| Required time for normalization (day) | 29.1±26.8 (4∼115) | 38.6±34.8 (3∼120) | 0.32 |
| Serum total bilirubin (mg/dl) | |||
| Preoperative | 2.6±1.8 (0.8∼7.1) | 2.6±2.3 (0.6∼11.7) | 0.98 |
| Postoperative 7 th day | 3.8±2.4 (1.8∼10.3) | 3.9±3.1 (1.0∼21.3) | 0.93 |
| Postoperative 1 st month | 1.6±1.4 (0.5∼5.9†) | 1.7±2.2 (0.4∼11.1‡) | 0.85 |
| Postoperative 3 rd month | 1.2±1.4 (0.7∼7.0‡) | 1.2±1.5 (0.6∼8.0‡) | 0.94 |
| Peak | 8.8±5.6 (4.0∼28.3) | 9.0±4.6 (4.0∼20.8) | 0.87 |
| Required time for normalization (day) | 23.6±22.1 (1∼95) | 31.1±37.4 (6∼160) | 0.42 |
| Prothrombin time (%) | |||
| Preoperative | 60.7±14.2 (35.0∼106.1) | 65.2±18.2 (33.2∼108.0) | 0.37 |
| Postoperative 7 th day | 70.2±9.7 (53.9∼87.8) | 78.8±11.8 (52.5∼112.5) | 0.12 |
| Postoperative 1 st month rd | 87.3±18.8 (57.6†∼135.7) | 96.8±16.4 (74.2∼128.0) | 0.09 |
| Postoperative 3 rd month | 89.6±15.9 (79.3∼123.8) | 98.6±12.3 (77.6∼119.7) | 0.05 |
| Required time for normalization (day) | 9.4±11.8 (3.0∼53.0) | 4.9±2.0 (2.0∼9.0) | 0.11 |
Table 6.
Comparison of hemagglutinin titer and anti-CD19 anti-body population between group I and II




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download