Abstract
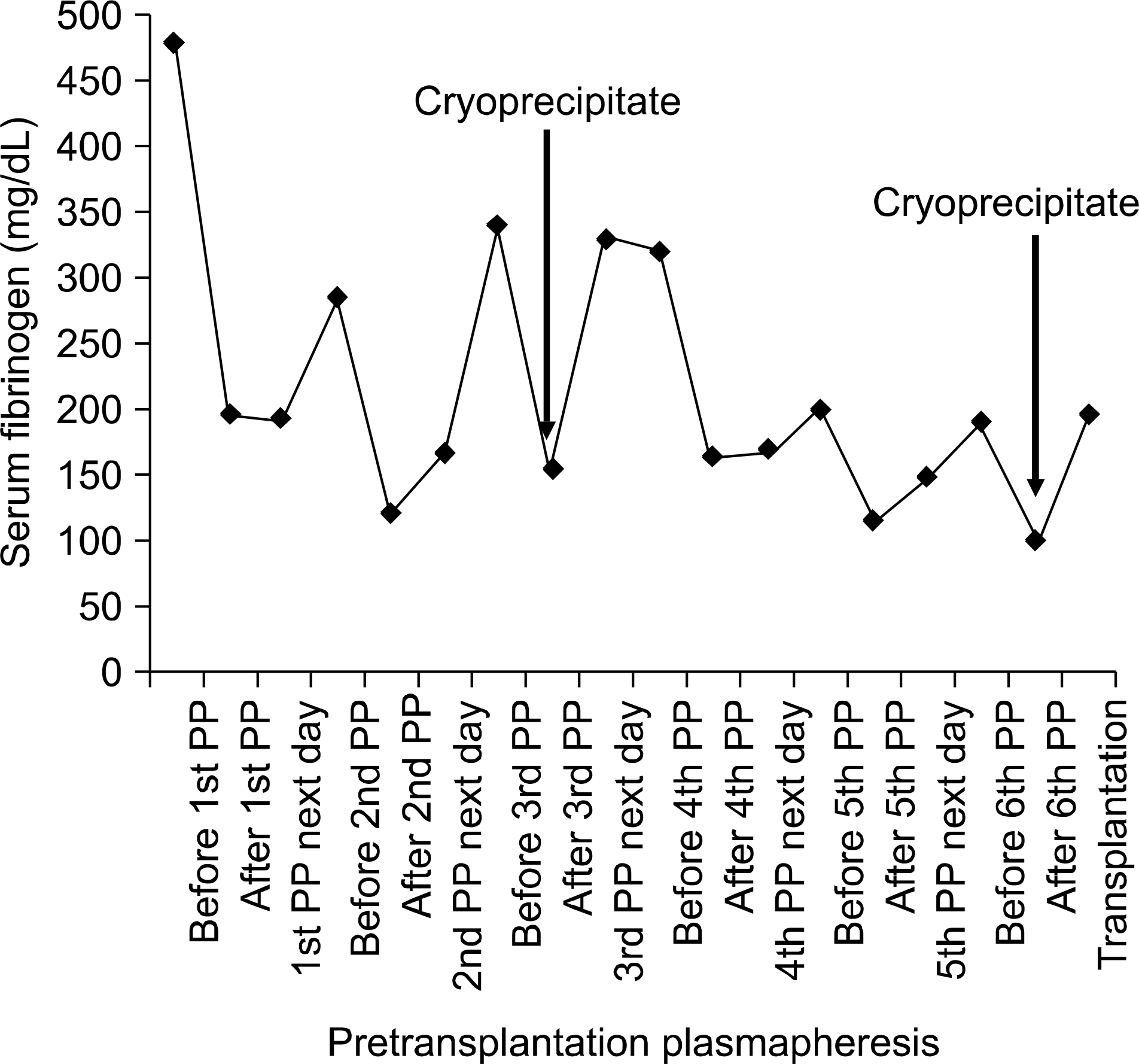
ABO-incompatible kidney transplantations have been performed successfully in Korea without splenectomy using plasmapheresis, anti-CD20 monoclonal antibody infusions and other immunosuppressants. However, there is no report of a case of ABO-incompatible kidney transplantation in a Jehovah’ s Witness. Hence, we report our experience of successful ABO-in-compatible kidney transplantation without blood products in a Jehovah’ s Witness. The recipient was treated with six sessions of plasmapheresis and he received intravenous rituximab before transplantation. Immunosuppressive regimen consisted of tacrolimus, mycophenolate mofetil and steroid. The replacement fluid for plasmapheresis was 5% albumin solution instead of fresh frozen plasma. We measured the clotting factors before and after plasmapheresis and used cryoprecipitate to prevent bleeding.
Go to : 
References
1). Kong JM, Lee DR, Jeong JH, Choi JH, Lee JO, Lee WR, et al. ABO blood group incompatible living donor kidney transplantation without splenectomy. J Korean Soc Transplant. 2009; 23:71–6. (공진민, 이동렬, 정준헌, 최재호, 이정오, 이화림, 등. 비장적출을 하지 않은 ABO 혈액형 부적합 생체 신장이식 경험. 대한이식학회지 2009;23: 71–6.).
2). Tydén G, Gunilla K, Helena G, John S, Torbjorn L and Ingella F. ABO-incompatible kidney transtrantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005; 5:145–8.
3). Takahashi K, Saito K. Present status of ABO-incompat-ible kidney transplantation in Japan. Xenotransplantation. 2006; 13:118–22.

4). Wilpert J, Fischer KG, Pisarski P, Wiech T, Daskalakis M, Ziegler A, et al. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010; 25:3778–86.

5). Wilpert J, Geyer M, Teschner S, Schaefer T, Pisarski P, Schulz-Huotari C, et al. ABO-incompatible kidney transplantation-proposal of an intensified apheresis strategy for patients with high initial isoagglutinine titers. J Clin Apher. 2007; 22:314–22.

6). Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompat-ible renal transplantation without splenectomy. Am J Transplant. 2004; 4:1315–22.

7). Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006; 6:2418–28.

8). Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007; 84(12 Suppl):S4–7.

9). Tydén G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, et al. Implementation of a Protocol for ABO-incompatible kidney transplantation-a three-center experience with 60 consecutive transplantations. Transplantation. 2007; 83:1153–5.
10). Szczepiorkowski ZM, Shaz BH, Bandarenko N, Winters JL. The new approach to assignment of ASFA catego-ries-introduction to the fourth special issue: clinical applications of therapeutic apheresis. J Clin Apher. 2007; 22:96–105.

11). Roback JD. Technical manual. 16th ed.Bethesda: American Association of Blood Bank;2008. p. 697–705.
12). Holt S, Donaldson H, Hazlehurst G, Varghese Z, Contreras M, Kingdon E, et al. Acute transplant rejection induced by blood transfusion reaction to the Kidd blood group system. Nephrol Dial Transplant. 2004; 19:2403–6.

13). Wood L, Jacobs P. The effect of serial therapeutic plasmapheresis on platelet count, coagulation factors, plasma immunoglobulin, and complement levels. J Clin Apher. 1986; 3:124–8.

14). Karlsson M, Ternström L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion. 2008; 48:2152–8.

15). Danés AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008; 94:221–6.

16). O'Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, et al. .;. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryopre-cipitate and cryosupernatant. Br J Haematol. 2004; 126:11–28.
17). Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev. 2009; 23:177–88.

18). Brecher ME. Technical Manual. 15th ed.Batheda: American Association of Blood Bank;2005. p. 502–503.
19). Muramoto O. Recent developments in medical care of Jehovah's Witnesses. West J Med. 1999; 170:297–301.
20). Park KY, Park CH, Lee HJ, Lim CY, Kwon JH, Ahn TH, et al. Cardiac transplantation in a Jehovah's wittness: a case report. Korean J Thorac Cardiovasc Surg. 1997; 30:537–9. (박국양, 박철현, 이현재, 임창영, 권전형, 안태호, 등. 여호와의 증인의 심장이식 1례 보고. 대한흉부외과학회지 1997;30: 537–9.).
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download