Abstract
Background
At the initiation of immunologic response, platelets rapidly release chemical mediators which may induce rejection of transplanted organ. The purpose of this study was to investigate the effect of antiplatelet agents in murine cardiac and skin transplantation models.
Methods
In the minor major histocompatibility (MHC) mismatch model, BALB/c (H2d) mice underwent heart transplantation from B10.D2 (H2d) mice. In the major MHC mismatch model, CBA (H2k) mice were used as the recipients and C57BL/10 (H2b) mice as donors. The recipients were divided into four groups and each group was treated with distilled water (DW), sarpogrelate, cilostazol, or clopidogrel respectively. For skin transplantation, the recipients in the minor MHC mismatch model were divided into four groups similar to those in cardiac transplantation. The recipients in the major MHC mismatch model were divided into DW-treated and sarpogrelate-treated groups. All treatments were done by the per oral route of administration.
Results
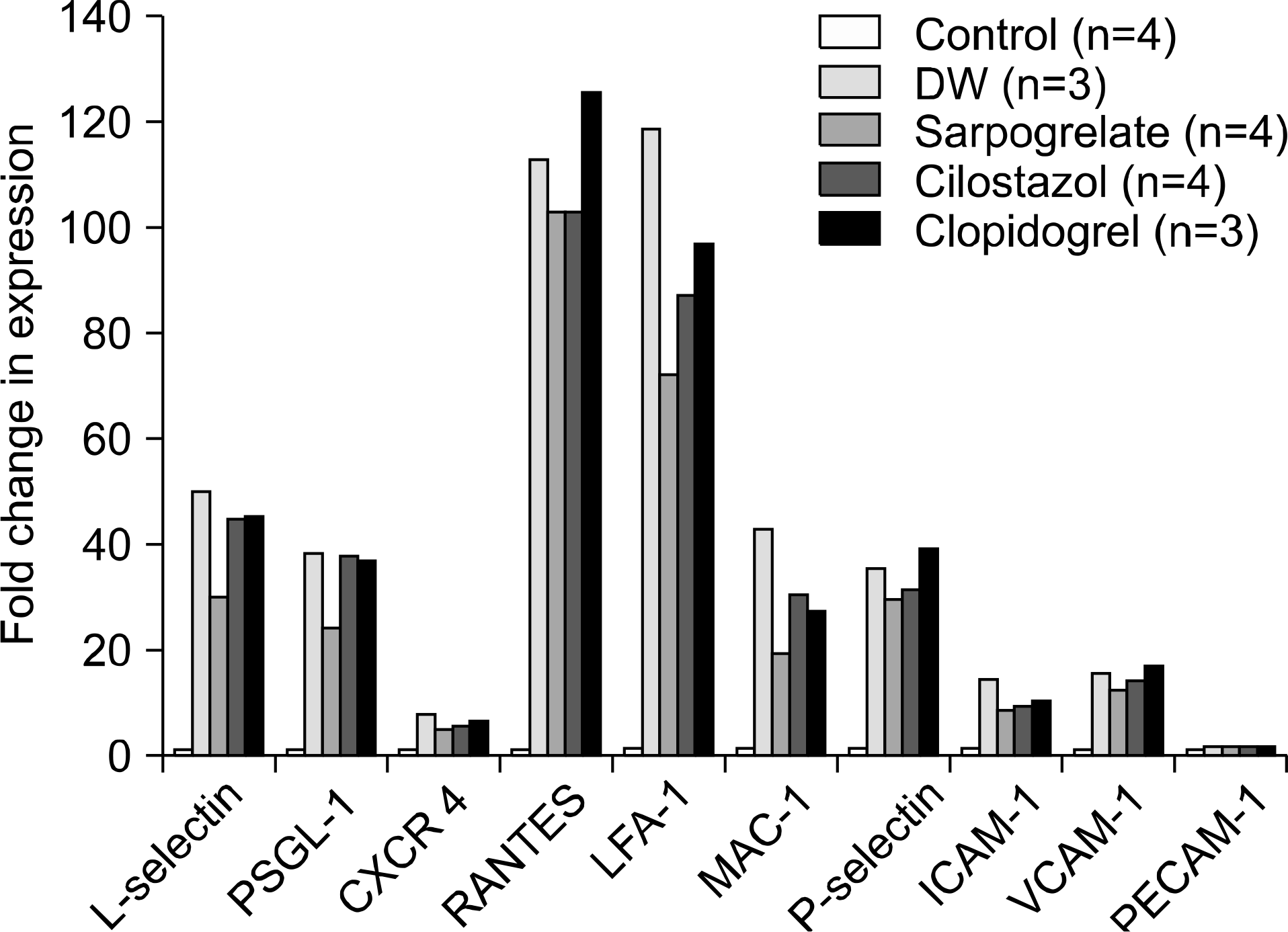
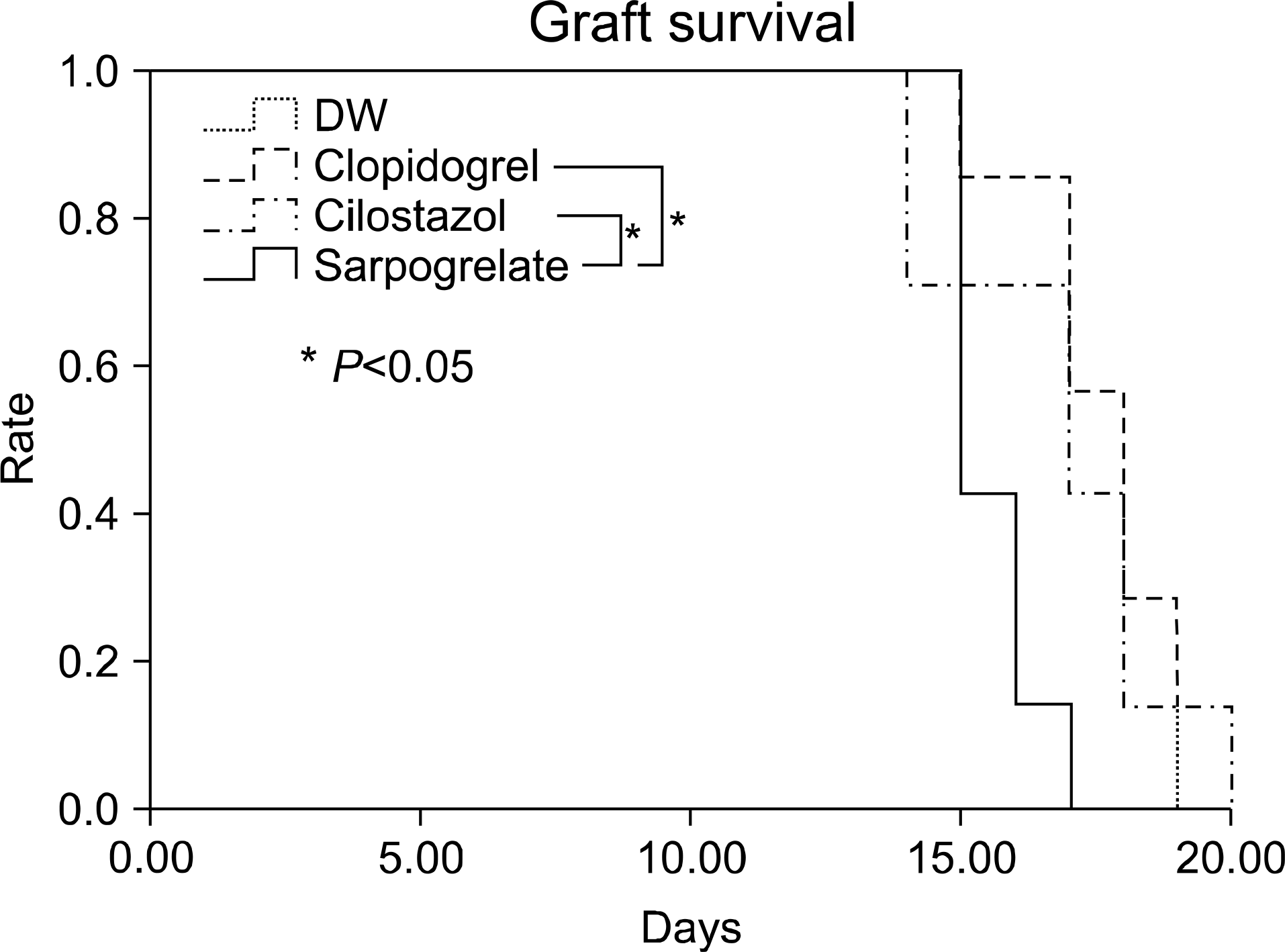
For graft survival in the minor MHC mismatch model of cardiac transplantation, sarpogrelate-treated group showed increased median survival time (MST) compared to the other groups (DW-treated group 17.5 days, sarpogrelate-treated group 88 days, cilostazol-treated group 13 days, clopidogrel-treated group 23 days). Similar results were observed in the major MHC mismatch model. In the major MHC mismatch model, the expression of adhesion molecules (L-selectin, intercellular adhesion molecule-1 [ICAM-1], Mac-1, lymphocyte function associated antigen-1 [LFA-1]) was significantly higher in DW-treated group compared to sarpogrelate-treated group (P<0.05) In the minor MHC mismatch model, MST in the antiplatelet-treated skin graft group was not remarkably prolonged compared to DW-treated group. In the major MHC mismatch model, sarpogrelate-treated group showed prolonged survival compared to DW-treated group (MST 25 vs. 19 days, P<0.05). There was no statistically significant difference in the proportion of activated T cells and regulatory T cells.
References
1). Graff J, Harder S, Wahl O, Scheuermann EH, Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther. 2005; 78:468–76.

2). Barradas MA, Mikhailidis DP. Serotonin, histamine and platelets in vascular disease with special reference to peripheral vascular disease. Braz J Med Biol Res. 1992; 25:1063–76.
3). Akiyoshi T, Zhang Q, Inoue F, Aramaki O, Hatano M, Shimazu M, et al. Induction of indefinite survival of fully mismatched cardiac allografts and generation of regulatory cells by sarpogrelate hydrochloride. Transplantation. 2006; 82:1051–9.

4). Wasowska BA, Qian Z, Cangello DL, Behrens E, van Tran K, Layton J, et al. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001; 71:727–36.
5). Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM 3rd. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002; 169:4620–7.

6). Ota H, Fox-Talbot K, Hu W, Qian Z, Sanfilippo F, Hruban RH, et al. Terminal complement components mediate release of von Willebrand factor and adhesion of platelets in arteries of allografts. Transplantation. 2005; 79:276–81.

7). Meehan SM, Limsrichamrern S, Manaligod JR, Junsanto T, Josephson MA, Thistlethwaite JR, et al. Platelets and capillary injury in acute humoral rejection of renal allografts. Hum Pathol. 2003; 34:533–40.

8). Morrell CN, Sun H, Swaim AM, Baldwin WM 3rd. Platelets an inflammatory force in transplantation. Am J Transplant. 2007; 7:2447–54.

9). Czapiga M, Kirk AD, Lekstrom-Himes J. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp Hematol. 2004; 32:135–9.

10). Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006; 116:769–74.

11). Porter KA. Renal transplantation. Heptinstall RH, editor. Pathology of the Kidney. 4th ed.Philadelphia, USA: Lippincott Raven;1992. p. 1799–931.
12). Lowenhaupt R, Nathan P. Platelet accumulation observed by electron microscopy in the early phase of renal allotransplant rejection. Nature. 1968; 220:822–5.
14). Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25-T cells. J Clin Invest. 2003; 112:1437–43.
15). Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling KL, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005; 35:1681–91.

16). Gahmberg CG, Tolvanen M, Kotovuori P. Leukocyte ad-hesion–structure and function of human leukocyte be-ta2-integrins and their cellular ligands. Eur J Biochem. 1997; 245:215–32.
17). Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005; 9:255–66.

18). Dunne JL, Collins RG, Beaudet AL, Ballantyne CM, Ley K. Mac-1, but not LFA-1, uses intercellular adhesion mole-cule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J Immunol. 2003; 171:6105–11.
19). Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010; 120:4520–31.

20). Arai K, Sunamura M, Wada Y, Takahashi M, Kobari M, Kato K, et al. Preventing effect of anti-ICAM-1 and an-ti-LFA-1 monoclonal antibodies on murine islet allograft rejection. Int J Pancreatol. 1999; 26:23–31.

21). Doggrell SA. Sarpogrelate: cardiovascular and renal clinical potential. Expert Opin Investig Drugs. 2004; 13:865–74.

22). León-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007; 109:3139–46.
Fig. 1.
Graft survival of minor mismatched cardiac transplantation (Donor: B10.D2, Recipient: BALB/c). Abbreviation: DW, distilled water.
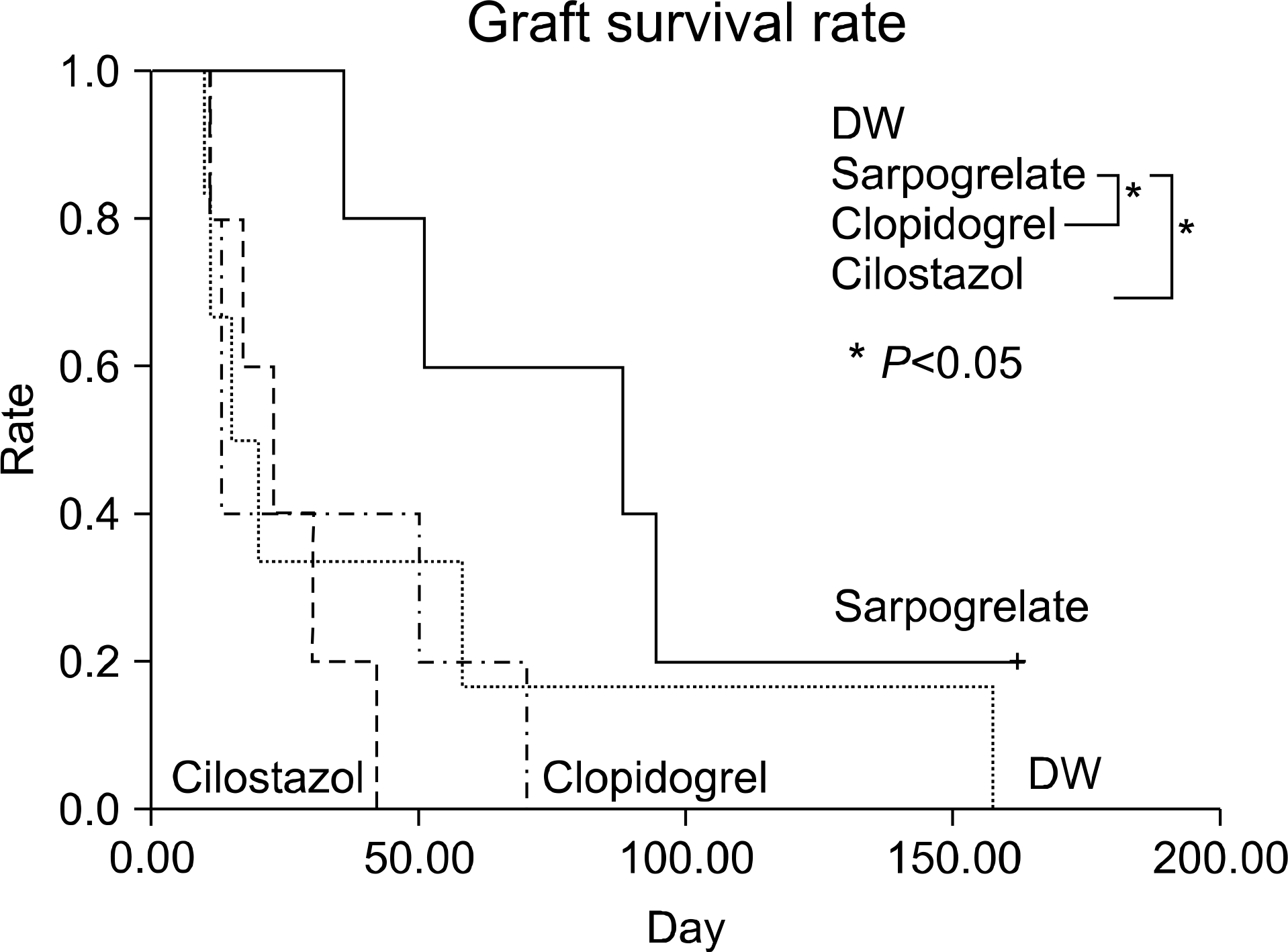
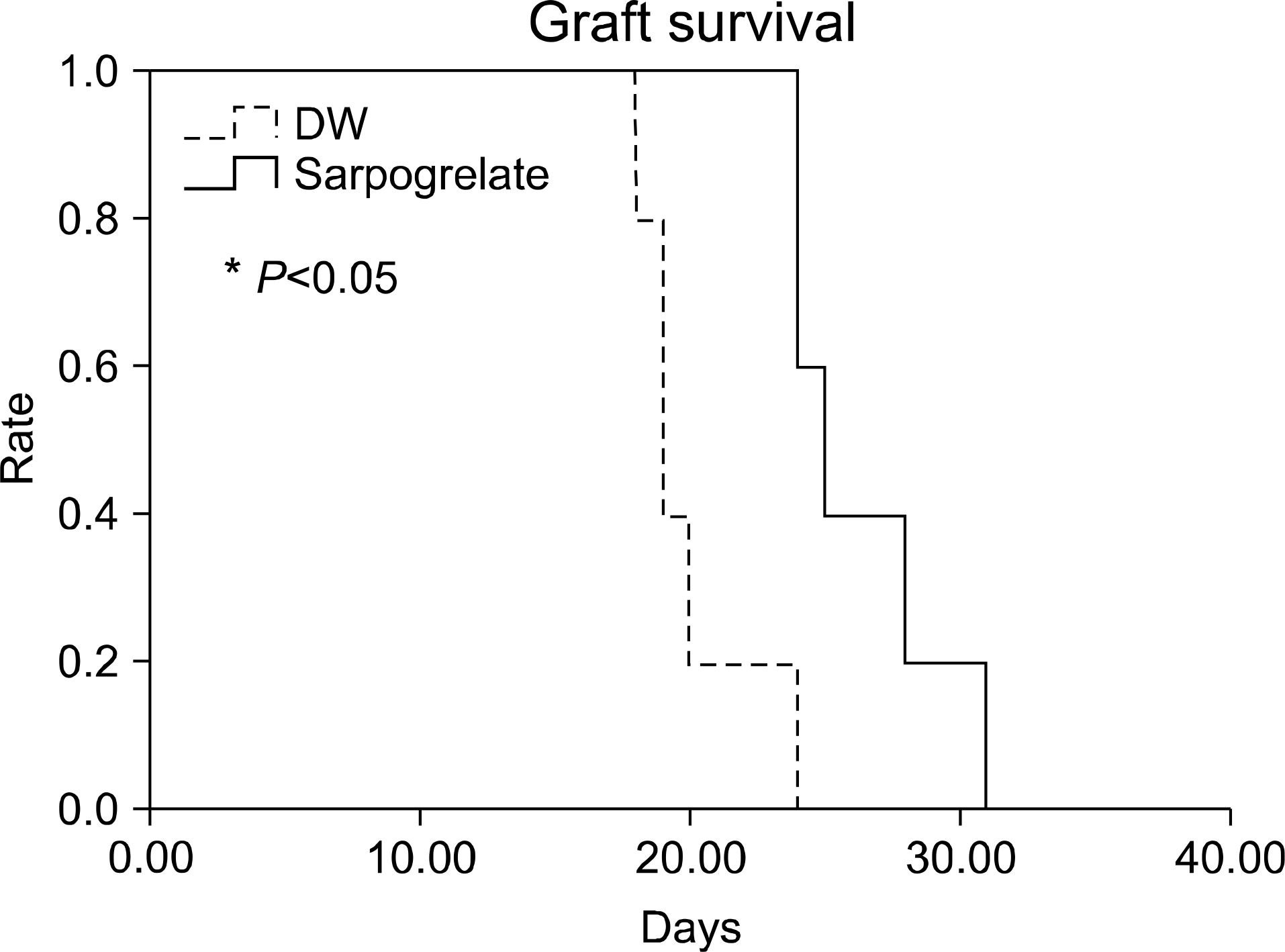
Fig. 2.
Graft survival of major mismatched cardiac transplantation (Donor: C57BL/10, Recipient: CBA). Abbreviation: DW, distilled water.
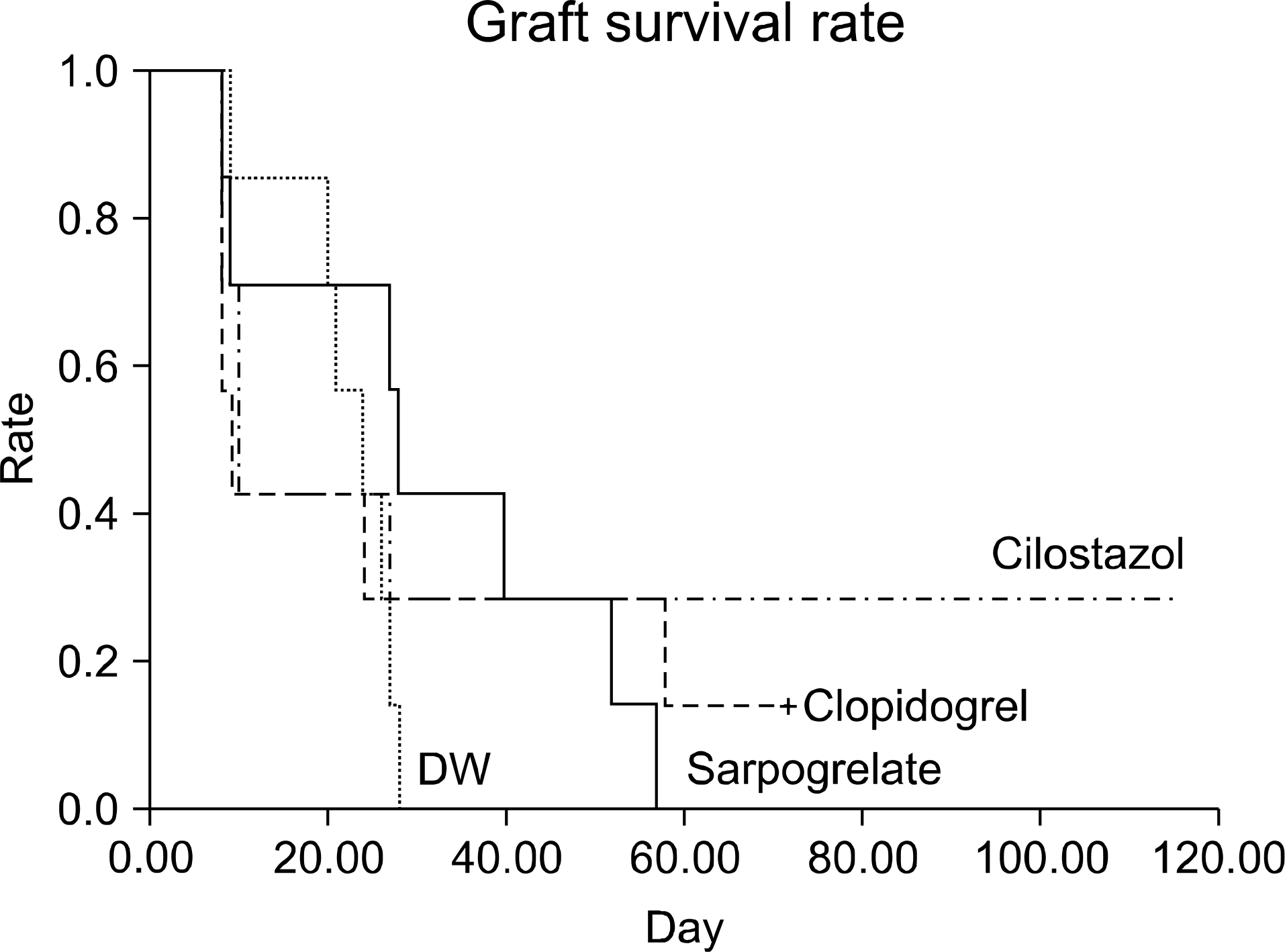
Fig. 3.
Quantitative RT-PCR of adhesion molecules and chemo-attractive cytokine. Abbreviation: DW, distilled water.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download