Abstract
Background
Several studies reported that sub-clinical rejection (SCR) detected by a protocol biopsy soon after renal transplantation does permanent damage to a renal allograft, contributing to chronic allograft nephropathy (CAN). This article investigated the risk factors involved in SCR and the effects of treating SCR, and evaluated the clinical significance of a protocol biopsy soon after renal transplantation.
Methods
From January 2007 to June 2010, 253 patients received renal transplantation. Patients were divided into two groups according to whether or not they had undergone a protocol biopsy. To analyze the effect of SCR treatments, patients who were diagnosed with SCR were divided into two groups according to whether or not they had been treated with SCR. The patients who did not undertake a protocol biopsy were included in the untreated groups.
Results
Among 138 patients who undertook protocol biopsies, 65 patients (47.1%) showed SCR. In univariate analysis, both the number of HLA-DR mismatches (P=0.003) and not using Simulect (P=0.01) were identified as risk factors of SCR. In multivariate analysis, not using Simulect (P=0.006) was identified as an risk factor independent of SCR. δ GFR, subtracting GFR at 1 week from GFR at that point, showed significant differences between SCR-treated patients and untreated patients at 1, 3, 6, 9, 12, 24, and 36 months with a P value of less than 0.05.
Go to : 
References
1). Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation. 2004; 78:242–9.

2). Morath C, Ritz E, Zeier M. Protocol biopsy: what is the rationale and what is the evidence? Nephrol Dial Transplant. 2003; 18:644–7.

3). Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006; 6:747–52.

4). Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003; 349:2326–33.

5). Min SI, Yun IJ, Kang JM, Park YJ, Min SK, Ahn C, et al. Moderate-to-severe early-onset hyperuricaemia: a prognostic marker of longterm kidney transplant outcome. Nephrol Dial Transplant. 2009; 24:2584–90.

6). Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–23.

7). hoi BS, Shin MJ, Shin SJ, Kim YS, Choi YJ, Kim YS, et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten year experience at a single center. Am J Transplant. 2005; 5:1354–60.
8). Furness PN, Philpott CM, Chorbadjian MT, Nicholson ML, Bosmans JL, Corthouts BL, et al. Protocol biopsy of the stable renal transplant: a multicenter study of methods and complication rates. Transplantation. 2003; 76:969–73.

9). Wilczek HE. Percutaneous needle biopsy of the renal allograft. A clinical safety evaluation of 1129 biopsies. Transplantation. 1990; 50:790–7.
10). Masin-Spasovska J, Spasovski G, Dzikova S, Petrusevska G, Lekovski Lj, Ivanovski N, et al. Do we have to treat subclinical rejections in early protocol renal allograft bi-opsies? Transplant Proc. 2007; 39:2550–3.

11). Rush D, Nickerson P, Gough J, McKenna R, Grimm P, Cheang M, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. 1998; 9:2129–34.

12). Rush D. Protocol biopsies for renal transplantation. Saudi J Kidney Dis Transpl. 2010; 21:1–9.
13). Beimler J, Zeier M. Borderline rejection after renal transplantation – to treat or not to treat. Clin Transplant. 2009; 23(Suppl 21):19–25.

14). Kee TY, Chapman JR, O'Connell PJ, Fung CL, Allen RD, Kable K, et al. Treatment of subclinical rejection diagnosed by protocol biopsy of kidney transplants. Transplantation. 2006; 82:36–42.

Go to : 
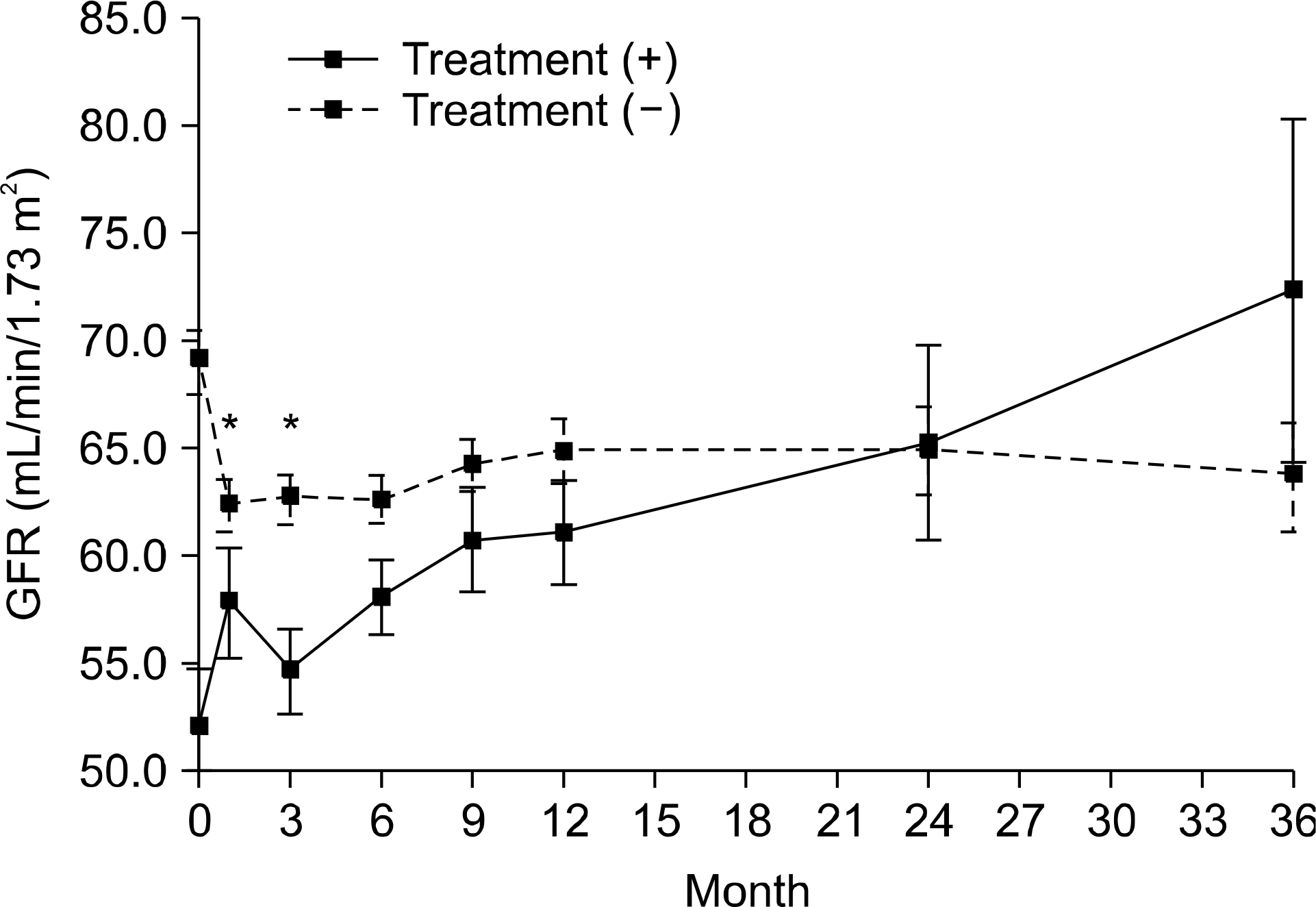 | Fig. 1.Changes in mean MDRD GFR of each group. There were no significant differences between two groups in GFR after 3 months. *P<0.05. |
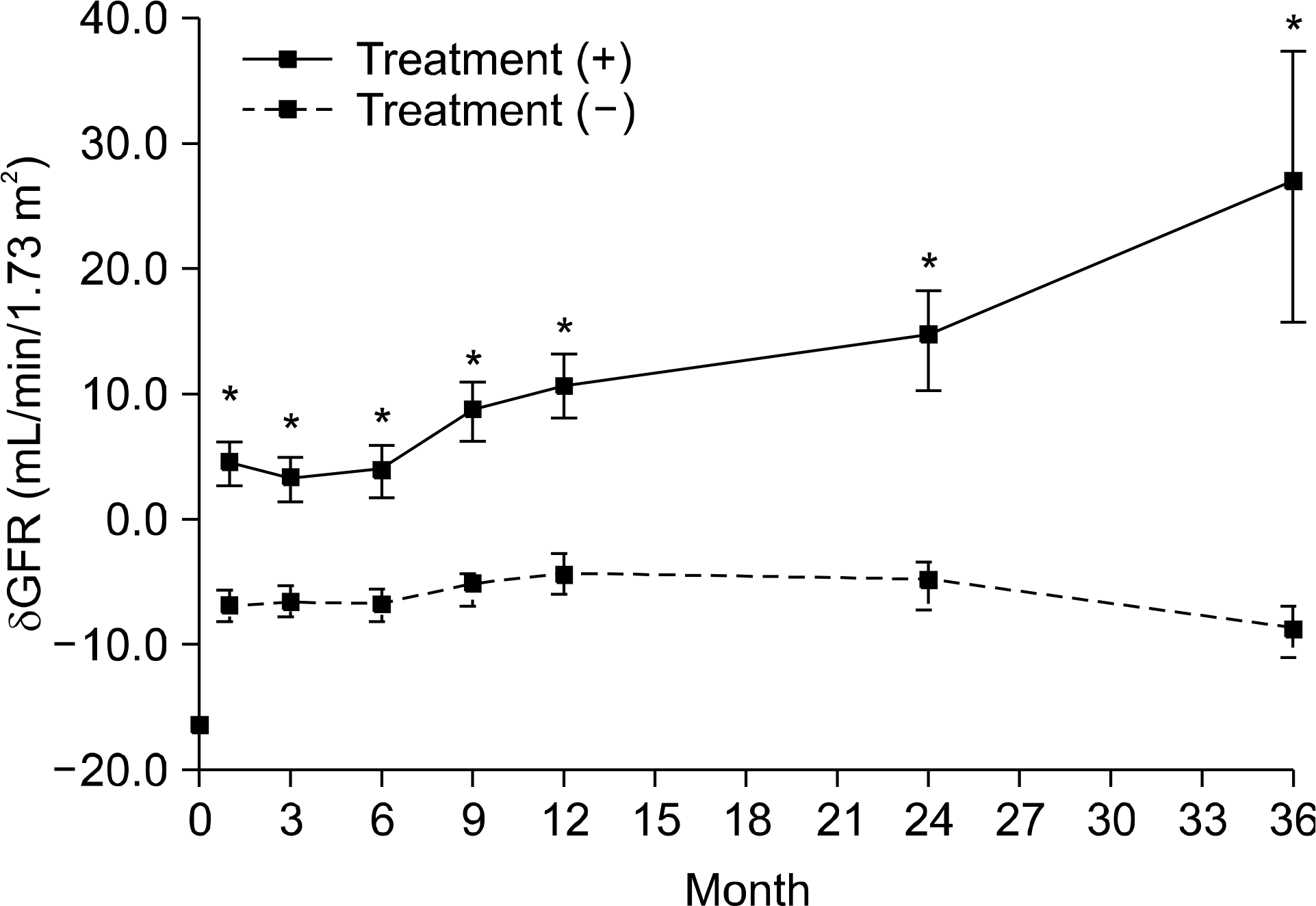 | Fig. 2.δ GFR defines as a result of subtraction MDRD GFR at 1 wk from MDRD GFR at the point. There were significant differences between two groups in δ GFR at 1, 3, 6, 9, 12, 24 and 36 months. *P<0.05. |
Table 1.
Demographics
Table 2.
SCR prevalence in early protocol biopsy
| No. of patients | Prevalence (%) | Accumulative prevalence (%) | ||
|---|---|---|---|---|
| SCR | AMR | 2 | 1.4 | 1.4 |
| Borderline change | 46 | 33.3 | 34.8 | |
| TCR – IA or IB | 11 | 7.9 | 42.8 | |
| TCR – IIA or IIB | 6 | 4.3 | 47.1 | |
| NR | 73 | 52.9 | 100 | |
| Total | 138 | 100 | 100 |
Table 3.
Univariate analysis of risk factors for SCR
Table 4.
Multivariate analysis of risk factors for SCR: multiple logistic regression




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download