Abstract
Background
In Korea, the number of liver transplantation (LT) center is still changing. Many more centers are performing liver transplantations than that during the past decades. But several centers have stopped liver transplantation, while some centers have newly started performing liver transplantation. We present our initial experience in a newly built center as an example for any center that is considering performing LT.
Methods
A total of 33 consecutive adult LTs that were performed from June 2006 to October 2009 were analyzed by comparing the first 11 living donor liver transplants (LDLTs) performed with the help of an outside experienced team (group 2) with the second 11 LDLTs (group 3) and the 11 deceased donor liver transplantations (DDLTs) cases (group 1) that were independently performed in our center.
Results
There was no operative mortality for the donors and there were two operative mortalities for the recipients. During a mean follow-up of 27.1 months (range: 2 days∼61 months), there were two cases of late mortality for the recipients. There were no re-operations and no major complications for the donors. The warm ischemic time was significantly longer in group 1 than that in groups 2 and 3. Otherwise, there was no significant difference in the operative outcomes among the three groups.
Go to : 
References
1). Starzl TE, Marchioro TL, Porter KA. Progress in homotransplantations of the liver. Adv Surg. 1966; 2:295–370.
2). Korean Network for Organ Sharing (KONOS). 2009 Annual Data Report [Internet]. Seoul: KONOS;2010. : 12 [cited 2011 Jun 28]. Available from:. http://konos.go.kr.
3). Mayo Clinic. The MELD Model, UNOS Modification [INTERNET]. Available from:. http://www.mayoclinic.org.
4). Kim S, Choi YS, Choi WH, Chang SH, Yun IJ, Song EY. A living donor liver transplantation after therapeutic plasmapheresis in a patient with positive HLA crossmatch. Korean J Blood Transfus. 2007; 18:260–4. (김세림, 최영숙, 최원혁, 장성환, 윤익진, 송은영. HLA 교차 시험 양성 환자에서 혈장교환술 후 시행한 생체 간이식 1예. 대한수혈학회지 2007;18: 260–4.).
5). Kim DK, Kim HK, Kim TY, Lim JA, Kim YL, Jang SH. Anesthesia for liver transplantation in a patient with hepatic failure combined with primary renal failure: A case report. Korean J Anesthesiol. 2007; 53:547–53. (김덕경, 김혜경, 김태엽, 임정애, 김양렬, 장성환. 원발성 신부전이 동반된 간부전 환자의 간이식술의 마취 관리-증례보고. 대한마취과학회지 2007;53: 547–53.).
6). Kim DK, Kim HK, Lim JA, Jeong SM, Jang SW, Yun IJ. Perioperative combined use of Sildenafil and inhaled Iloprost for moderate portopulmonary hypertension in a patient undergoing liver transplantation: A case report. Korean J Anesthesiol. 2008; 54:102–8. (김덕경, 김혜경, 임정애, 정승민, 장성환, 윤익진. 주술기 Sildenafil과 흡입용 Iloprost 병용처치를 통한 중등도 문맥 폐동맥 고혈압 환자의 간이식 마취 경험. 대한마취과학회지 2008;54: 102–8.).
7). Kim SH, Cho SY, Park SJ, Lee KW, Han SS, Lee SA, et al. Learning curve for living-donor liver transplantation in a fledgling cancer center. Transpl Int. 2009; 22:1164–71.

8). Green M, Wald ER, Tzakis A, Todo S, Srarzl TE. Aspergillosis of the CNS in a pediatric liver transplant recipient: case report and review. Rev Infect Dis. 1991; 13:653–7.

9). Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–9.

10). Lee SG, Lee YJ, Kwon TW, Park KM, Choi KM, Min PC, et al. Unlimited-term passive immunoprophylaxis after liver transplantation in HBsAg-positive patients. Transplant Proc. 1996; 28:1176–7.
11). Lee SK, Park JH, Joh JW, Kim SJ, Choi IS, Choi SH, et al. Prophylaxis aginst hepatitis B recurrence following liver transplantation in HBsAg (+) patients. Transplant Proc. 2000; 32:2248–9.
12). Chang SH, Suh KS, Yi NJ, Choi SH, Lee HJ, Seo JK, et al. Active immunization against de novo hepatitis B virus infection in pediatric patients after liver transplantation. Hepatology. 2003; 37:1329–34.

13). Kwon CH, Suh KS, Yi NJ, Chang SH, Cho YB, Cho JY, et al. Long-term protection against hepatitis B in pediatric liver recipients can be achieved effectively with vaccination after transplantation. Pediatr Transplant. 2006; 10:479–86.

14). Middleton PF, Duffield M, Lynch SV, Padbury RT, House T, Stanton P, et al. Living donor liver transplantation–adult donor outcomes: a systematic review. Liver Transpl. 2006; 12:24–30.

Go to : 
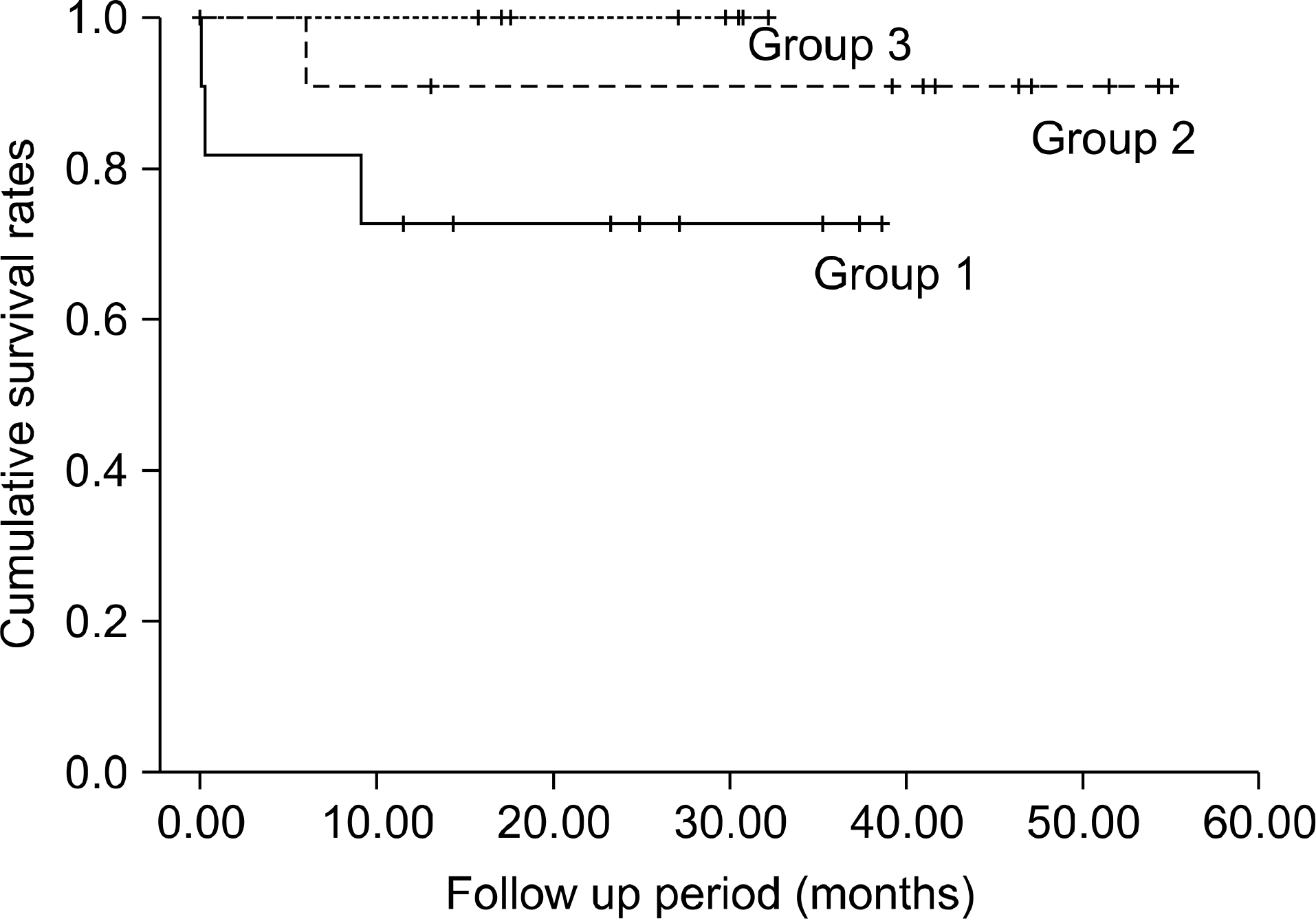 | Fig. 1.Cumulative survival rates of the three groups. There was no significant difference among the three groups (P-value= 0.071). |
Table 1.
Clinical characteristics of recipients
Table 2.
Outcomes of recipients
Table 3.
Characteristics and outcomes of living donors




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download