Abstract
Background
Luminex panel reactive antibody (PRA) is a method that is well known for its high sensitivity and specificity. By using a single antigen assay, the presence or absence of donor specific antibody (DSA) can be determined and its strength can be quantified in terms of the mean fluorescence intensity (MFI). In this study, we analyzed the correlation between the pre-transplant PRA and DSA measured by the Luminex method and the post-transplant clinical features after kidney transplantation.
Methods
A total of 123 pre-transplant sera samples from kidney transplanted patients were tested. Luminex-PRA identification tests were performed using a Luminex fluoroanalyzer and a LifeCodes class I, II ID Kits. Single antigen assay by the Luminex method was used for detecting DSA and its MFI.
Results
The positive Luminex-PRA group included more highly-sensitized patients such as women, patients with a previously positive lymphocyte cross match test and patients who were undergoing retransplantation. There was no correlation between the acute rejection rate and positive PRA on the Luminex-PRA. However, pretransplant DSA detected by the single antigen assay was significantly associated with episodes of antibody mediated rejection (P=0.047, OR=10.2), and DSA with higher MFI values (MFI≥3,000) was associated with antibody mediated rejection (P=0.023).
Go to : 
References
1). Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969; 280:735–9.

2). Tanaka N, Takasugi M, Terasaki PI. Presensitization to transplants detected by cellular immunity tests. Transplantation. 1971; 12:514–8.

3). Terasaki PI, Kreisler M, Mickey RM. Presensitization and kidney transplant failures. Postgrad Med J. 1971; 47:89–100.

4). Worthington JE, Thomas AA, Dyer PA, Martin S. Detection of HLA-specific antibodies by PRA-STAT and their association with transplant outcome. Transplantation. 1998; 65:121–5.

5). Schonemann C, Groth J, Leverenz S, May G. HLA class I and class II antibodies: monitoring before and after kidney transplantation and their clinical relevance. Transplantation. 1998; 65:1519–23.
6). Amico P, Honger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-spe-cific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009; 87:1681–8.

7). Jung S, Oh EJ, Yang CW, Ahn WS, Kim Y, Park YJ, et al. Comparative evaluation of ELISA and Luminex panel reactive antibody assays for HLA alloantibody screening. Korean J Lab Med. 2009; 29:473–80. (정선경, 오은지, 양철우, 안웅식, 김용구, 박연준, 등. HLA 동종항체 선별을 위한 ELISA 및 Luminex Panel Reactive Antibody 검사의 비교평가. 대한진단검사의학회지 2009;29: 473–80.).

8). Ki CS, Yang YS, Kim DW. Comparison of complement-dependent cytotoxicity, enzyme-linked immunosorbent assay and flow cytometric assay for the detection of HLA class I alloantibodies. Korean J Clin Pathol. 1998; 18:624–9. (기창석, 양윤선, 김대원. HLA Class I 동종항체 선별을 위한 보체의존림프구독성법, 효소면역측정법 및 유세포분석법에 의한 Panel Reactive Antibody 검사법의 비교. 대한임상병리학회지 1998;18: 624–9.).
9). Oh EJ, Lee J, Yang CW, Moon IS, Park YJ, Han K. Comparison of anti-HLA detecting methods; cytotoxicity, flow cytometric crossmatch, multiple antigen-ELISA, single antigen-ELISA. J Korean Soc Transplant. 2008; 22:5–91. (오은지, 이제훈, 양철우, 문인성, 박연준, 한경자. 항-HLA 항체 검출을 위한 검사방법의 비교: Cytotoxicity, Flow Cytometric Crossmatch, Multiple Antigen-ELISA, and Single Antigen-ELISA. 대한이식학회지 2008;22: 5–91.).
10). El-Awar N, Lee J, Terasaki PI. HLA antibody identification with single antigen beads compared to conventional methods. Hum Immunol. 2005; 66:989–97.

11). Colombo MB, Haworth SE, Poli F, Nocco A, Puglisi G, Innocente A, et al. Luminex technology for anti-HLA antibody screening: evaluation of performance and of impact on laboratory routine. Cytometry B Clin Cytom. 2007; 72:465–71.

12). Oh EJ, Park YJ, Kim JY, Yang CW, Kim DG, Moon IS. Detection of donor specific anti-HLA antibodies using antibody monitoring system. J Korean Soc Transplant. 2006; 20:63–8. (오은지, 박연준, 김진영, 양철우, 김동구, 문인성. Antibody Monitoring System을 이용한 공여자 특이 HLA 항체 검출. 대한이식학회지 2006;20: 63–8.).
13). Muro M, Llorente S, Marin L, Moya-Quiles MR, Gonzalez-Soriano MJ, Prieto A, et al. Acute vascular rejection mediated by HLA antibodies in a cadaveric kidney recipient: discrepancies between FlowPRA, ELISA and CDC vs luminex screening. Nephrol Dial Transplant. 2005; 20:223–6.
14). Riethmuller S, Ferrari-Lacraz S, Muller MK, Raptis DA, Hadaya K, Rusi B, et al. Donor-specific antibody levels and three generations of crossmatches to predict antibody-mediated rejection in kidney transplantation. Transplantation. 2010; 90:160–7.
Go to : 
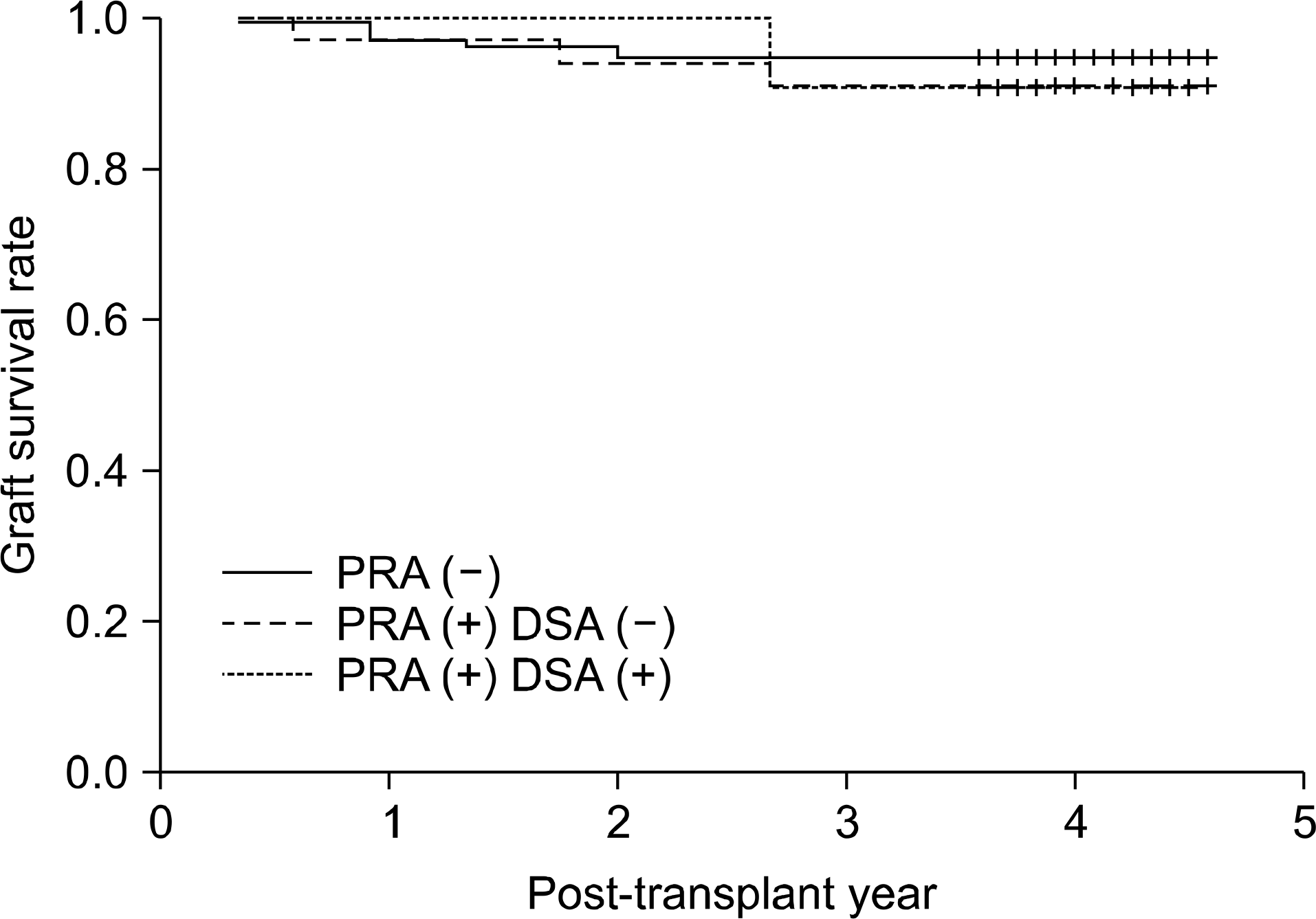 | Fig. 1.Graft survival rate according to pre-transplant panel reactive antibody (PRA) and donor specific antibody (DSA). Abbreviations: PRA, panel reactive antibody measured by Luminex-PRA test; DSA, donor specific antibody measured by Luminex-Single antigen PRA test. |
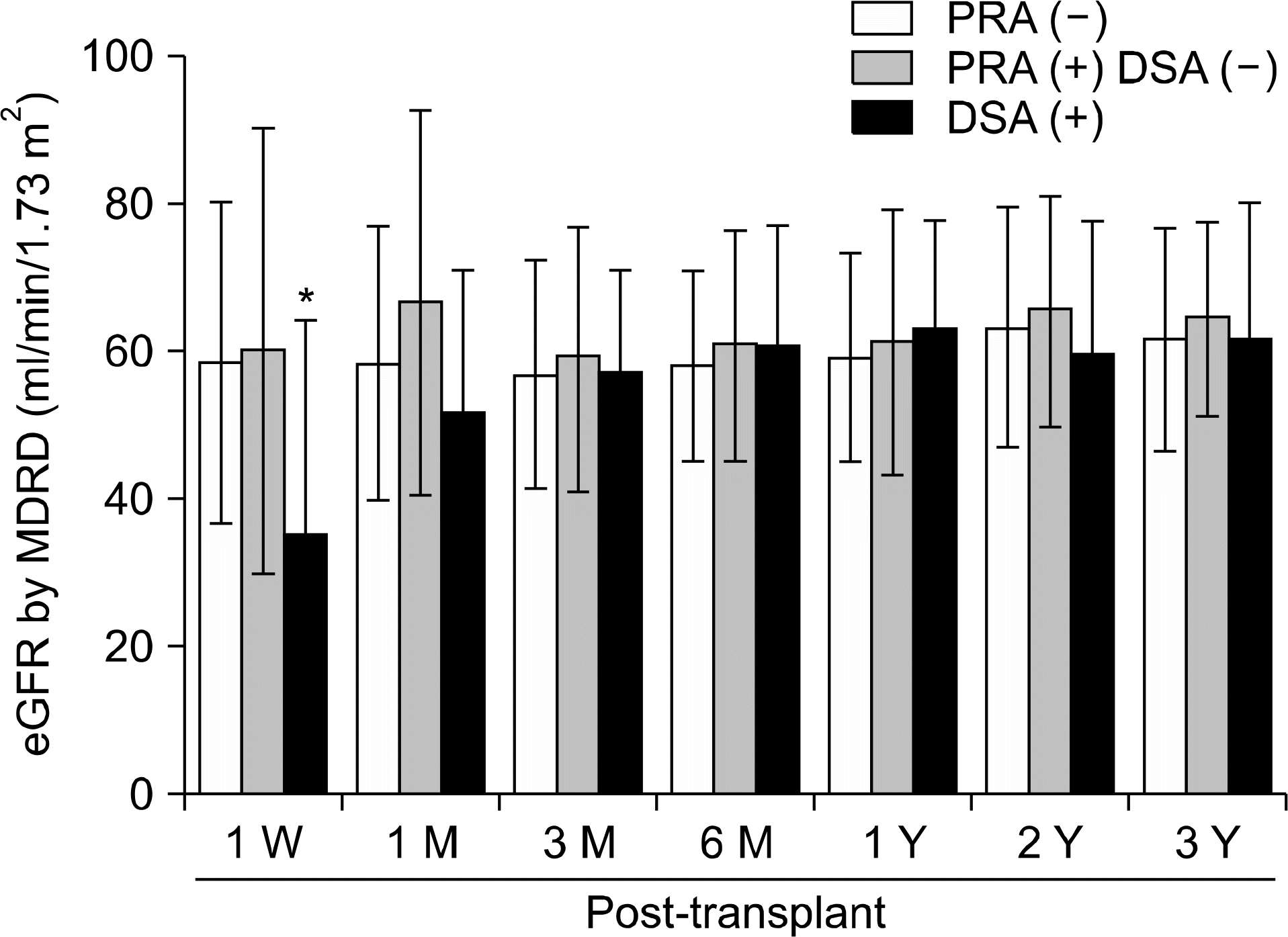 | Fig. 2.Post-transplant change of graft function measured by estimated glomerular filtration rate (eGFR, by MDRD formula) according to the pre-transplant panel reactive antibody (PRA) and donor specific antibody (DSA) status. Abbreviations: PRA, panel reactive antibody measured by Luminex-PRA test; DSA, donor specific antibody measured by Luminex-Single antigen PRA test. *P<0.05. |
Table 1.
Association between clinical factors related with HLA alloimmunization and panel reactive antibody (PRA) results by Luminex method
Table 2.
Association between acute rejection episodes and panel reactive antibody (PRA) results by Luminex method
| Clinical factors | Luminex-PRA ID | P-value∗ | ||
|---|---|---|---|---|
| I (-) and II (-) (n=78) | I or II (+) (n=27) | I (+) and II (+) (n=18) | ||
| Rejection-free (n=98) | 64 (65.3%) | 21 (21.4%) | 13 (13.3%) | |
| Clinical rejection∗ (n=9) | 4 (44.4%) | 2 (22.2%) | 3 (33.3%) | 0.201 |
| Pathologic rejection, cell-mediated (n=11) | 7 (63.6%) | 4 (36.4%) | 0 (-) | 0.340 |
| Pathologic rejection, antibody-mediated (n=5) | 3 (60.0%) | 0 (-) | 2 (40.0%) | 0.237 |
| Abbreviations: I, class I; II, class II. | ||||
Table 3.
Association between acute rejection episodes and pre-transplant donor specific antibody (DSA) status
| Clinical factors | DSA (-)(n=112) | DSA (+)(n=11) | P-value∗ |
|---|---|---|---|
| Rejection-free (n=98) | 92 (93.9%) | 6 (6.1%) | |
| Clinical rejection† (n=9) | 7 (77.8%) | 2 (22.2%) | 0.135 |
| Pathologic rejection, | 10 (90.9%) | 1 (9.1%) | 0.536 |
| cell-mediated (n=11) | |||
| Pathologic rejection, | 3 (60.0%) | 2 (40.0%) | 0.047‡ |
| antibody-mediated (n=5) |
Table 4.
Association between acute rejection episodes and strength (MFI values) of donor specific antibody which was measured by single antigen assay




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download