Abstract
With advancements of liver transplantation, the patient’ s survival improved remarkably. Thus the long-term survivors are increasing especially for the pediatric liver transplantation recipients. Consequently they are facing the challenge of maintaining graft function while minimizing long-term complications. In this review, I will discuss calcineurin inhibitor toxicity, problems with steroid, adherence to medical regimen, posttransplant growth, chronic graft dysfunction, tolerance.
Go to : 
References
1). Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963; 117:659–76.
2). Kim KM, Oh SH, Lee YJ, Kim T, Kim DY, Lee SG. Clinical experience of over 200 cases of pediatric liver transplantation in a single center: Improved patient survival. Liver Transpl. 2011; 17(Suppl 1):S233.
3). Devlin J, Williams R, Neuhaus P, McMaster P, Calne R, Pichlmayr R, et al. Renal complications and development of hypertension in the European study of FK 506 and cyclosporin in primary liver transplant recipients. Transpl Int. 1994; 7(Suppl 1):S22–6.

4). Menegaux F, Keeffe EB, Andrews BT, Egawa H, Monge H, Concepcion W, et al. Neurological complications of liver transplantation in adult versus pediatric patients. Transplantation. 1994; 58:447–50.

5). Varo E, Padin E, Otero E, Tomé S, Castroagudin JF, Delgado M, et al. Cardiovascular risk factors in liver allograft recipients: relationship with immunosuppressive therapy. Transplant Proc. 2002; 34:1553–4.

6). Allman SD, McWhorter AG, Seale NS. Evaluation of cyclosporin-induced gingival overgrowth in the pediatric transplant patient. Pediatr Dent. 1994; 16:36–40.
7). Al-Sinani S, Dhawan A. Corticosteroids usage in pediatric liver transplantation: To be or not to be! Pediatr Transplant. 2009; 13:160–70.
8). Oh SH, Kim KM, Kim DY, Lee YJ, Rhee KW, Jang JY, et al. Long-term outcomes of pediatric living donor liver transplantation at a single institution. Pediatr Transplant. 2010; 14:870–8.

9). Cho JM, Kim KM, Oh SH, Lee YJ, Rhee KW, Yu E. De novo autoimmune hepatitis in Korean children after liver transplantation: A single institution's experience. Transplant Proc. 2011; 43:2394–6.

10). Gras JM, Gerkens S, Beguin C, Janssen M, Smets F, Otte JB, et al. Steroid-free, tacrolimus-basiliximab immunosuppression in pediatric liver transplantation: clinical and pharmacoeconomic study in 50 children. Liver Transpl. 2008; 14:469–77.

11). Dew MA, Dabbs AD, Myaskovsky L, Shyu S, Shellmer DA, DiMartini AF, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009; 88:736–46.

12). Ekong UD, Bhagat H, Alonso EM. Once daily calcineurin inhibitor monotherapy in pediatric liver transplantation. Am J Transplant. 2010; 10:883–8.

13). Park SJ, Rim SH, Kim KM, Lee JH, Choi BH, Lee SY, et al. Long-term growth of pediatric patients following living-donor liver transplantation. J Korean Med Sci. 2005; 20:835–40.

14). Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997; 63:243–9.
Go to : 
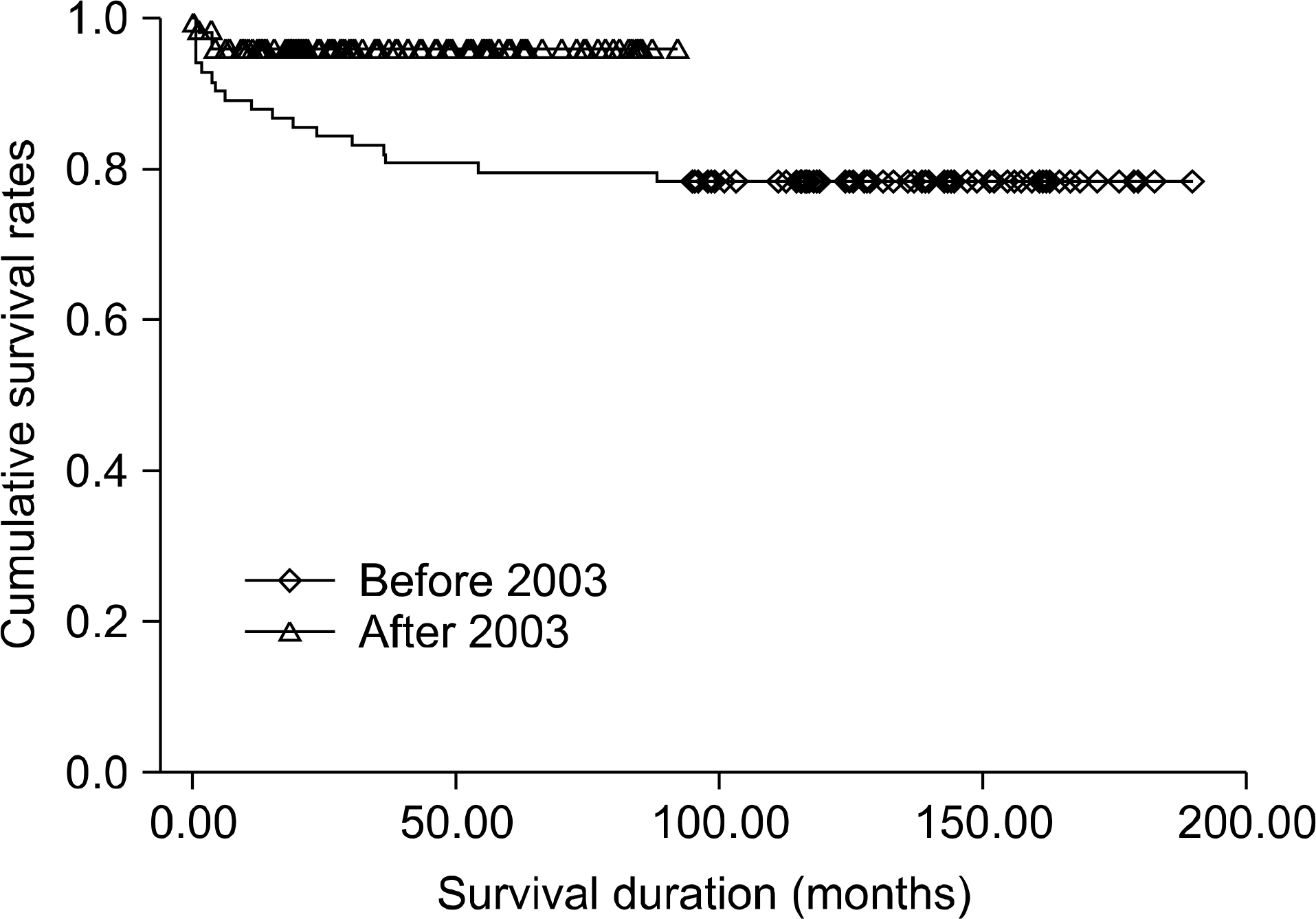 | Fig. 1.The overall rates of patient survival at two time period. Those before 2003 at 1 and 5 years were 86.4%, 79.5%, and 78.4%, respectively. Those after 2003 were 95.4% and 95.4%, respectively (P<0.05, Kaplan-Meier method). There is a statistically significant improvement in patient survival. Reprinted from reference [2]. |
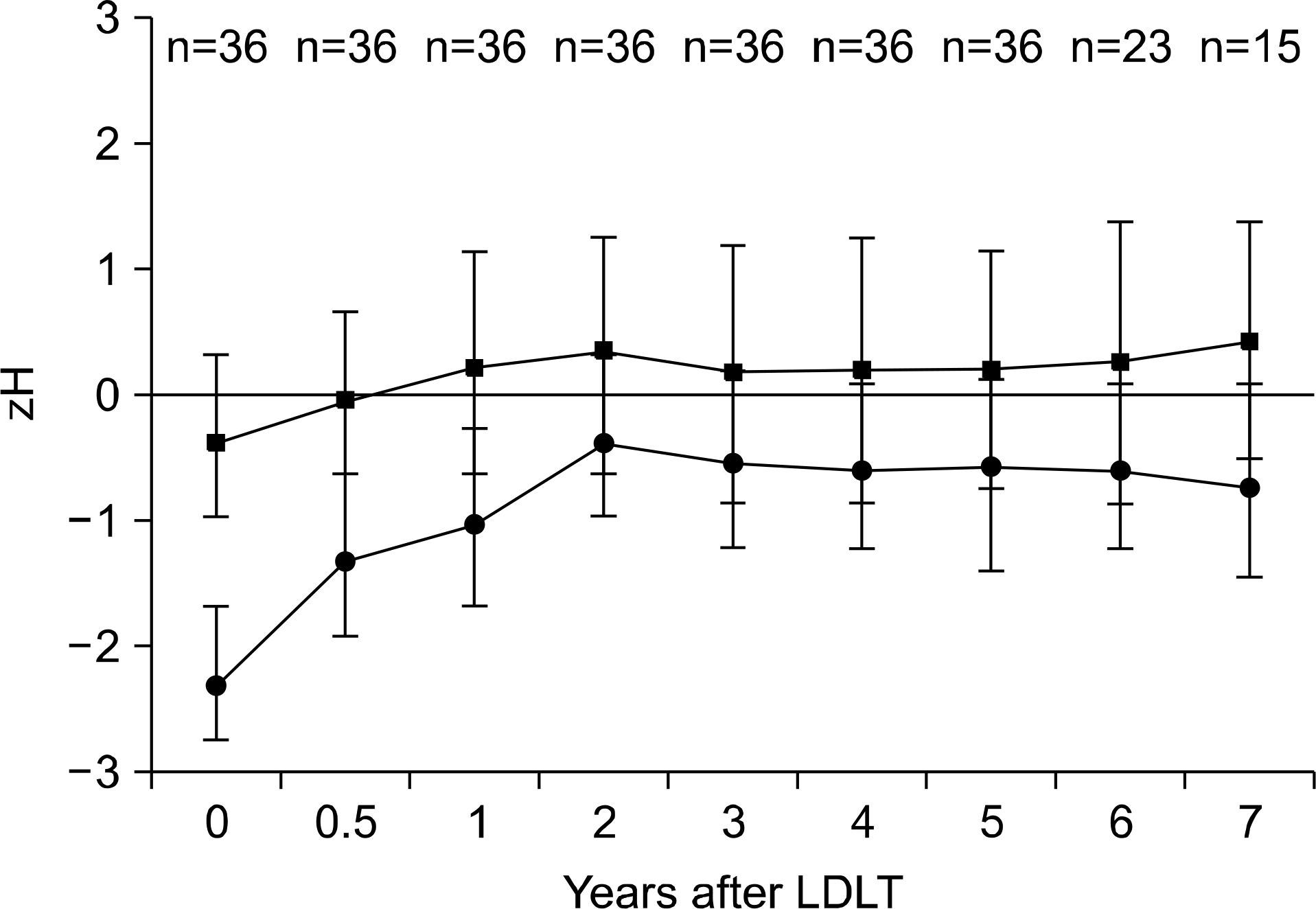 | Fig. 2.Post-transplantation height mean standard deviation scores (zH) of children with retarded growth (●) and normal growth (■) at time of transplantation. The height of growth-re-tarded children was restored by catch-up growth and non-growth-retarded children grew adequately for up to 7 years. Adapted from reference [13]. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download