Abstract
Acute antibody-mediated rejection (AMR) developing simultaneously with acute cellular rejection has been rarely reported as a long-term complication of renal transplantation, and it can present on top of another chronic pathology affecting the graft. A 51-year-old female patient with chronic kidney disease of unknown etiology received renal transplantation 12 years ago from a living unrelated donor with 3 HLA mismatches. She received induction therapy with methylprednisolone and was maintained on steroids, mycophenolate mofetil and cyclosporine A (CsA). For a period of twelve years post-transplantation, she was clinically and biochemically stable. She presented with a rise in serum creatinine (SCr.) from 1.3 mg/dL to 2.4 mg/dL but did not have proteinuria. Graft biopsy revealed findings suggestive of acute cellular rejection on top of antibody-mediated rejection (type II) and chronic calcineurin inhibitor toxicity. Panel reactive antibody (PRA) test levels were 3.6%, 91.7% for class I and II respectively. The patient was treated with high-dose methylprednisolone for 3 days but serum creatinine was not fully normalised. After 2 weeks from initial methyl-PDS pulse therapy, she received intravenous immunoglobulin, plasma exchange and anti-CD20 (rituximab). Cyclosporine was changed to tacrolimus. She achieved a complete response, and SCr. was maintained at 1.3 mg/dL without proteinuria. Follow-up PRA test levels were 0%, 75% for class I and II. Current therapies have had considerable success in reversing mixed, acute humoral and cellular rejection since it is being identified quickly and treated aggressively. The best use of rituximab to treat AMR should be evaluated in controlled trials using dosing strategies that include longer courses or retreatment schedules.
References
2). Feucht HE. Complement C4d in graft capillaries – the missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003; 3:646–52.

3). Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria-an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003; 3:708–14.
4). Halloran PF. The clinical importance of alloantibody-mediated rejection. Am J Transplant. 2003; 3:639–40.

5). Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strat-egies? Am J Transplant. 2004; 4:1289–95.

7). Gautreau C, Suberbielle C, Andrade J, Charron D. Donor-specific antibody detection for diagnosis of antibody-mediated rejection in kidney transplantation. Clin Transpl. 2006. 502.
8). Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007; 18:1046–56.

9). Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002; 13:242–51.

10). Nickeleit V, Mihatsch MJ. Kidney transplants, antibodies and rejection: is C4d a magic marker? Nephrol Dial Transplant. 2003; 18:2232–9.

11). Yang CW, Oh EJ, Lee SB, Moon IS, Kim DG, Choi BS, et al. Detection of donor-specific anti-HLA class I and II antibodies using antibody monitoring system. Transplant Proc. 2006; 38:2803–6.

12). Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent longterm prognostic factor. J Am Soc Nephrol. 2002; 13:234–41.

13). Gibney EM, Cagle LR, Freed B, Warnell SE, Chan L, Wiseman AC. Detection of donor-specific antibodies using HLA-coated microspheres: another tool for kidney transplant risk stratification. Nephrol Dial Transplant. 2006; 21:2625–9.
14). Weinstein D, Braun WE, Cook D, McMahon JT, Myles J, Protiva D. Ultra-late antibody-mediated rejection 30 years after a living-related renal allograft. Am J transplant. 2005; 5:2576–8.

15). Poduval RD, Kadambi PV, Josephson MA, Cohn RA, Harland RC, Javaid B, et al. Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation. 2005; 79:228–35.

16). Kedainis RL, Koch MJ, Brennan DC, Liapis H. Focal C4d + in renal allografts is associated with the presence of donor-specific antibodies and decreased allograft survival. Am J Transplant. 2009; 9:812–9.
17). Chung BH, Jung HS, Jeon SH, Park YJ, Choi SO, Yoon JM, et al. Clinical significance of C4d-positivity in renal transplant recipients with acute rejection. J Korean Soc Transplant. 2005; 19:137–41. .(정병하, 정희선, 전상훈, 박용재, 최선욱, 윤정민, 등.급성 거부 반응을 보인 신 이식환자에서 C4d 양성의 임상적 의의. 대한이식학회지 2005;19;137–41.).
18). Watschinger B, Pascual M. Capillary C4d deposition as a marker of humoral immunity in renal allograft rejection. J Am Soc Nephrol. 2002; 13:2420–3.

19). Pascual M, Saidman S, Tolkoff-Rubin N, Williams WW, Mauiyyedi S, Duan JM, et al. Plasma exchange and tacro-limus-mycophenolate rescue for acute humoral rejection in kidney transplantation. Transplantation. 1998; 66:1460–4.
20). Crespo M, Lozano M, Sole M, Mila J, Esforzado N, Martorell J, et al. Diagnosis and treatment of acute humoral rejection after kidney transplantation: preliminary experience. Transplant Proc. 2003; 35:1677–8.

21). Ibernon M, Gil-Vernet S, Carrera M, Serón D, Moreso F, Bestard O, et al. Therapy with plasmapheresis and intravenous immunoglobulin for acute humoral rejection in kidney transplantation. Transplant Proc. 2005; 37:3743–5.
22). Jordan SC, Vo AA, Toyoda M, Tyan D, Nast CC. Posttransplant therapy with high-dose intravenous gam-maglobulin: Applications to treatment of antibody-media-ted rejection. Pediatr Transplant. 2005; 9:155–61.

23). Casadei DH, Rial MC, Raimondi E, Goldberg J, Argento J, Haas E. Complementary data about the inhibitory effects of intravenous immunoglobulins in vitro and in vivo. Transplantation. 1997; 63:1191–2.

24). Kubori T, Mezaki T, Kaji R, Kimura J, Hamaguchi K, Hirayama K, et al. The clinical usefulness of high-dose intravenous immunoglobulin therapy for chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy. No to Shinkei. 1999; 51:127–35.
25). Lewanska M, Siger-Zajdel M, Selmaj K. No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. Eur J Neurol. 2002; 9:565–72.

26). Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006; 19:621–8.

27). Pescovitz MD. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006; 6:859–66.

28). Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008; 359:242–51.

29). Faguer S, Kamar N, Guilbeaud-Frugier C, Fort M, Modesto A, Mari A, et al. Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation. 2007; 83:1277–80.

30). Shirakawa H, Ishida H, Shimizu T, Omoto K, Iida S, Toki D, et al. The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: a singlecenter experience. Clin Transplant. 2010 Dec 22. [Epub ahead of print].

31). Stasi R, Brunetti M, Stipa E, Amadori S. Selective B-cell depletion with rituximab for the treatment of patients with acquired hemophilia. Blood. 2004; 103:4424–8.

32). Lee SM, Joo SH, Kim JS, Goldstein MJ, Cohen DJ, Hardy MA. Rituximab rescue for refractory antibody mediated rejection after kidney transplantation. J Korean Soc Transplant. 2004; 18:140–3. (이삼열, 주선형, 김주섭, Goldstein MJ, Cohen DJ, Hardy MA. 신장이식 후의 항체매개성 거부반응 치료를 위한 Rituximab의 효과. 대한이식학회지 2004;18: 140–3.).
33). Michaels PJ, Fishbein MC, Colvin RB. Humoral rejection of human organ transplants. Springer Semin Immunopathol. 2003; 25:119–40.

34). Wong W, Lee RA, Saidman SL, Smith RN, Zorn E. Bortezomib in kidney transplant recipients with antibody mediated rejection: three case reports. Clin Transpl. 2009; 23:401–5.
Fig. 1.
Clinical course; MPDS pulse, 250 mg iv bid for 3days; plasmapheresis, 1 plasma volume, 5% albumin replacement solution, COBEⓇ Spectra Apheresis System; Rituximab 200 mg/body single infusion. Abbreviations: CsA, cyclosporine; MMF, mycophenolate mofetil; PDS, prednisone; Bx., biopsy; MPDS, methyl prednisolone; PP/IVIG, plasmapheresis with intravenous immune globulin.
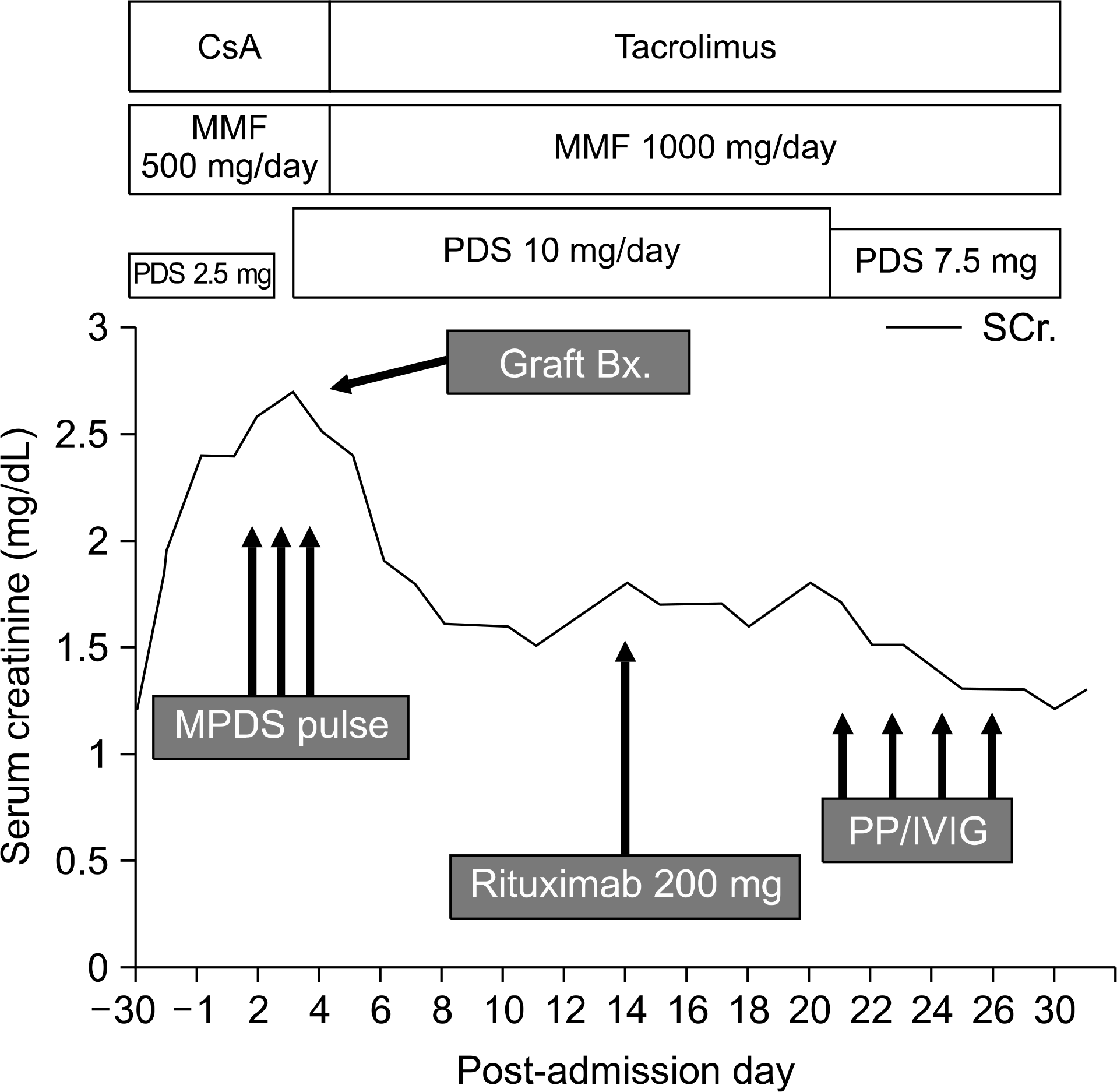
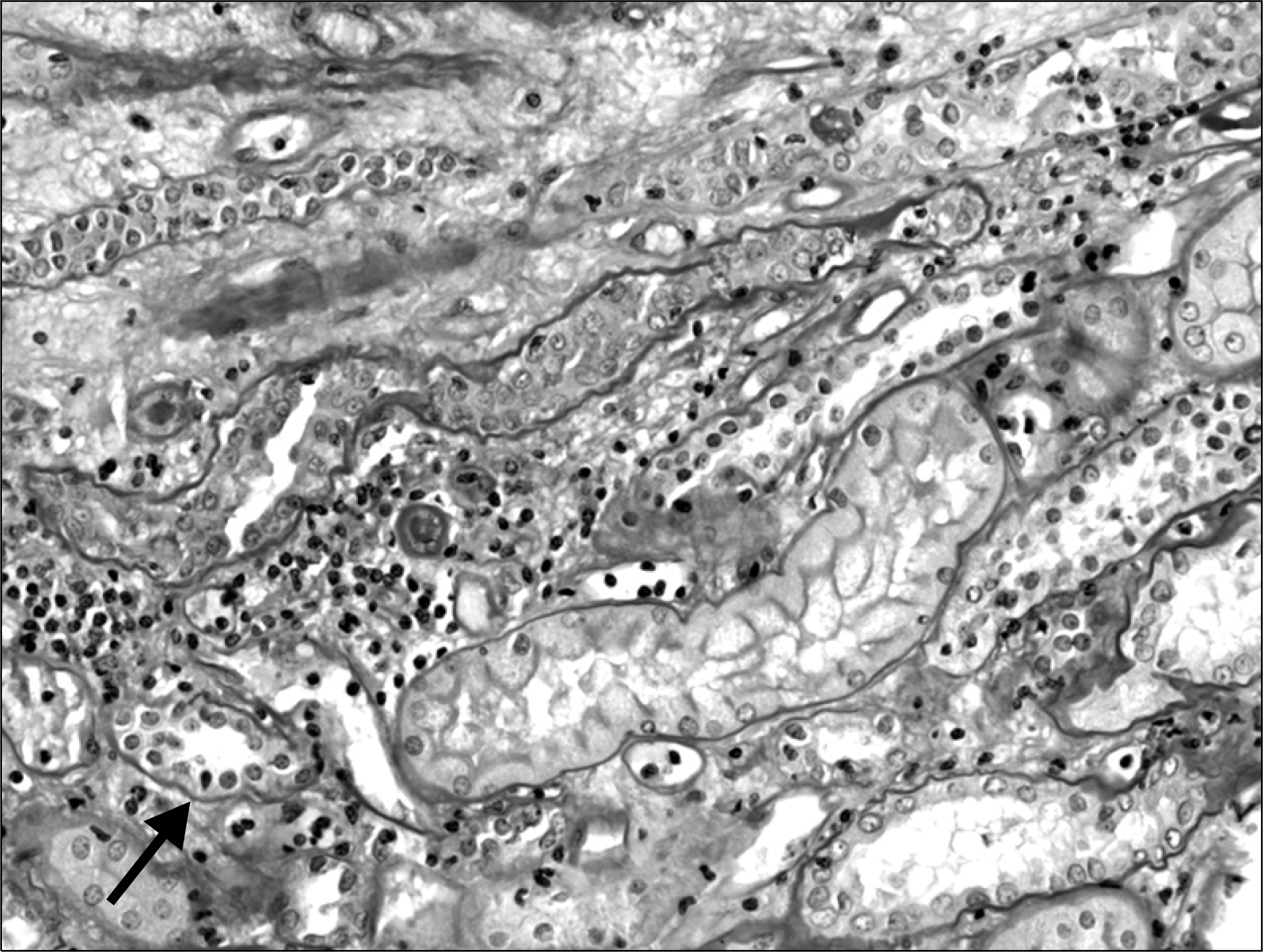
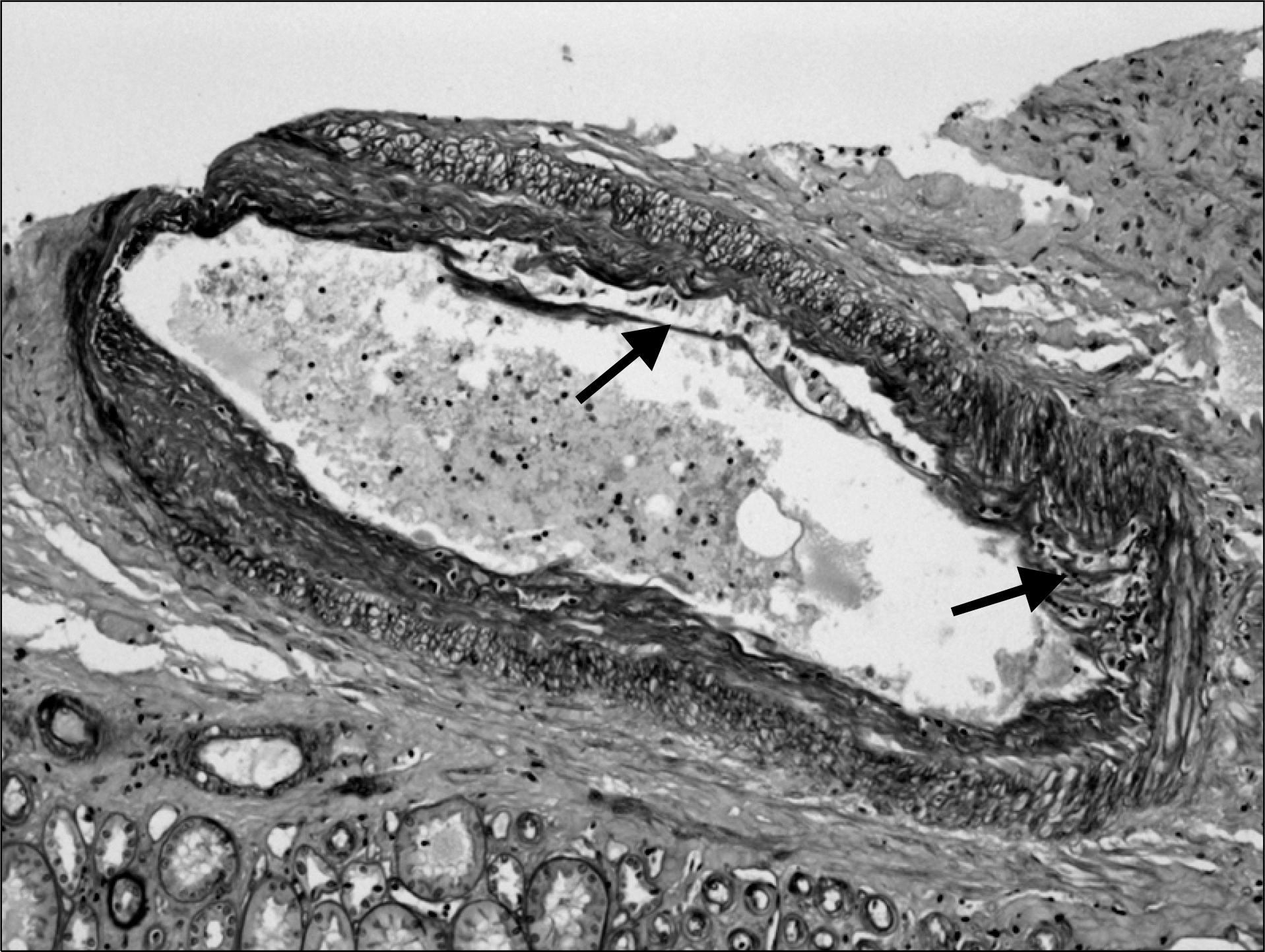
Fig. 2.
Light microscopically, there was mild to moderate intimal arteritis (PAS stain, original magnification ×400).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download