Abstract
Background
With advances in immunosuppression, graft and patient survival rates have increased significantly, but acute cellular rejection remains an important problem following liver transplantation (LT), and late acute rejection (LAR) occurs in a small percentage of recipients. Some risk factors for LAR have been identified, yet the cause of LAR has not been completely investigated. The efficacy of mycophenolate mofetil (MMF) administered in combination with calcineurin inhibitor (CNI) for reduction of LAR has been demonstrated.
Methods
Between January 2006 and August 2007, adult LT recipients (n=309) were enrolled in this study. Biopsy-proven acute rejection that occurred >6 months after LT was defined as LAR. The immunosuppression regimens, CNI or CNI plus MMF, were used continuously for at least 6 months after LT. The mean follow-up period was 34.8 months (range, 25∼46 months).
Results
LAR occurred in 17 cases (5.5%). The incidence of LAR in the CNI (n=138) or CNI plus MMF groups (n=171) was 8.6% (n=12) and 2.9% (n=5), respectively (P=0.015). Multivariate Cox regression confirmed that CNI plus MMF versus CNI therapy is associated with a decreased risk of LAR (relative risk, 0.33; P=0.04).
Go to : 
References
1). Demetris AJ, Ruppert K, Dvorchik I, Jain A, Minervini M, Nalesnik MA, et al. Real-time monitoring of acute liver-allograft rejection using the Banff schema. Transplantation. 2002; 74:1290–96.
2). Mor E, Gonwa TA, Husberg BS, Goldstein RM, Klintmalm GB. Late-onset acute rejection in orthotopic liver transplantation-associated risk factors and outcome. Transplantation. 1992; 54:821–4.

3). Ramji A, Yoshida EM, Bain VG, Kneteman NM, Scudamore CH, Ma MM, et al. Late acute rejection after liver transplantation: the Western Canada experience. Liver Transpl. 2002; 8:945–51.

4). Gavlik A, Demirbas A, Tsaroucha A, Webb MG, Nery JR, Khan MF, et al. Mycophenolate mofetil rescue therapy in liver transplant recipients: an extended follow-up. Transplant Proc. 1997; 29:2971–2.

5). Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995; 60:225–32.
6). Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001; 7:442–50.

7). Wiesner RH, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Lake JR. Mycophenolate mofetil combination therapy improves longterm outcomes after liver transplantation in patients with and without hepatitis C. Liver Transpl. 2005; 11:750–9.

8). Wiesner RH, Steffen BJ, David KM, Chu AH, Gordon RD, Lake JR. Mycophenolate mofetil use is associated with decreased risk of late acute rejection in adult liver transplant recipients. Am J Transplant. 2006; 6:1609–16.

9). Anand AC, Hubscher SG, Gunson BK, McMaster P, Neuberger JM. Timing, significance, and prognosis of late acute liver allograft rejection. Transplantation. 1995; 60:1098–103.

10). Cakaloglu Y, Devlin J, O'Grady J, Sutherland S, Portmann BC, Heaton N, et al. Importance of concomitant viral infection during late acute liver allograft rejection. Transplantation. 1995; 59:40–5.

11). Florman S, Schiano T, Kim L, Maman D, Levay A, Gondolesi G, et al. The incidence and significance of late acute cellular rejection (>1,000 days) after liver transplantation. Clin Transplant. 2004; 18:152–5.
12). Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999; 30:1121–7.

13). Narumi S, Roberts JP, Emond JC, Lake J, Ascher NL. Liver transplantation for sclerosing cholangitis. Hepatology. 1995; 22:451–7.

14). Akamatsu N, Sugawara Y, Tamura S, Keneko J, Matsui Y, Hasegawa K, et al. Late-onset acute rejection after living donor liver transplantation. World J Gastroenterol. 2006; 12:6674–7.

15). Soin AS, Rasmussen A, Jamieson NV, Watson CJ, Friend PJ, Wight DG, et al. CsA levels in the early posttransplant period-predictive of chronic rejection in liver transplantation? Transplantation. 1995; 59:1119–23.

16). Uemura T, Ikegami T, Sanchez EQ, Jennings LW, Narasimhan G, McKenna GJ, et al. Late acute rejection after liver transplantation impacts patient survival. Clin Transplant. 2008; 22:316–23.

17). Barkmann A, Nashan B, Schmidt HH, Böker KH, Emmanouilidis N, Rosenau J, et al. Improvement of acute and chronic renal dysfunction in liver transplant patients after substitution of calcineurin inhibitors by mycophenolate mofetil. Transplantation. 2000; 69:1886–90.

Go to : 
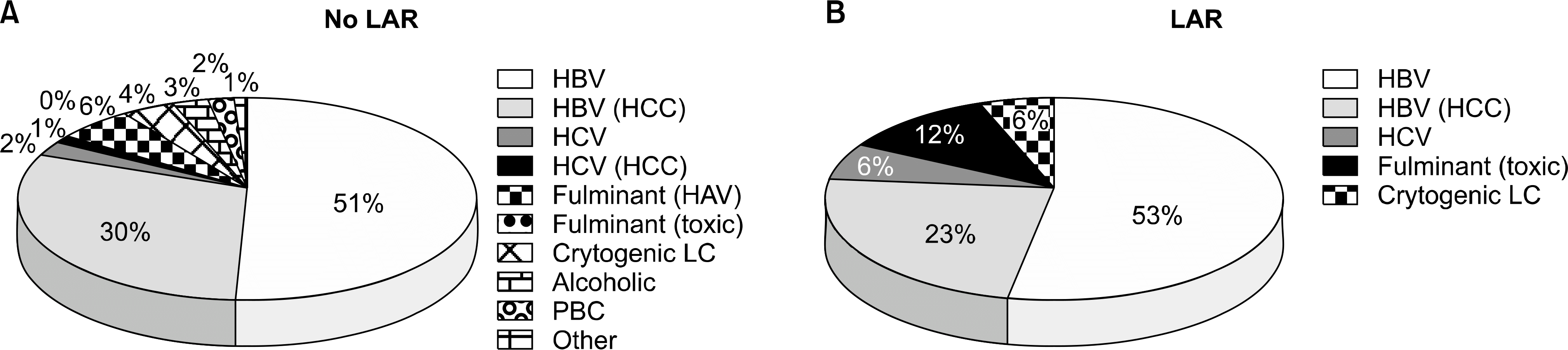 | Fig. 1.Etiology of liver disease. (A) Patients without late acute rejection (LAR). (B) Patients with LAR. Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LC, liver cirrhosis; PBC, primary biliary cirrhosis. |
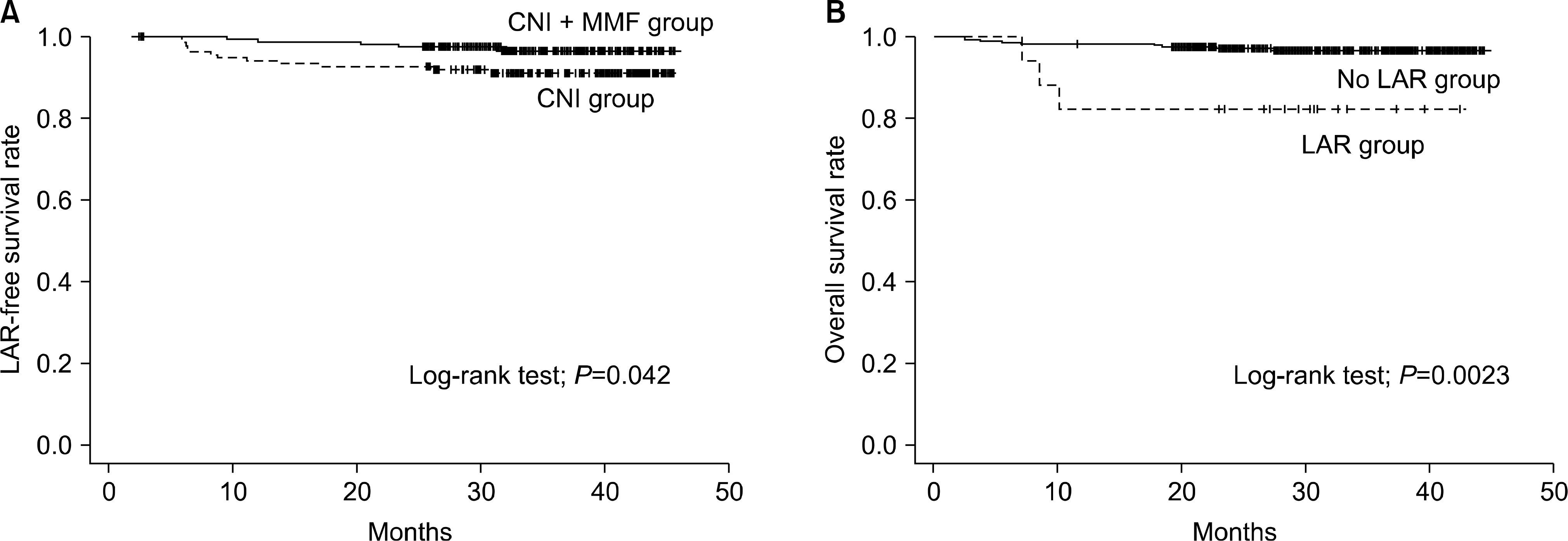 | Fig. 2.Kaplan-Meier analysis. (A) LAR-free survival rate between CNI and CNI+ MMF group (log-rank test; P=0.042). (B) Overall survival rate between LAR and No LAR group (log-rank test; P=0.0023). Abbreviations: LAR, late acute rejection; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil. |
Table 1.
Target trough levels of calcineurin inhibitors of our center
| Postoperative time (months) | Tacrolimus (ng/mL) | Cyclosporine (ug/mL) |
|---|---|---|
| 1 | 15∼16 | 150∼180 |
| 6 | 9∼11 | 100∼120 |
| 12 | 6∼8 | 80∼100 |
Table 2.
Baseline clinical characteristics
Table 3.
Immunosuppression levels at the time of 1, 6, 12 months follow-ups after liver transplantation
Table 4.
Comparison of CNI trough level in No late acute rejection group
| Months | No LAR | P | ||
|---|---|---|---|---|
| CNI | CNI+ MMF | |||
| FK (ng/mL) | 1 | 18.2 | 14.9 | 0.04 |
| (mean) | 6 | 9.3 | 7.9 | 0.02 |
| 12 | 7.6 | 7.1 | 0.65 | |
| CSA (ug/mL) | 1 | 143 | 186 | 0.36 |
| (mean) | 6 | 97 | 105 | 0.69 |
| 12 | 91 | 94 | 0.88 | |
Table 5.
Relative risk of LAR-free survival with Cox proportional hazard model (Univariate analyses of demographic characteristics)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download