Abstract
Hepatocellular carcinoma (HCC) has become an important indication for liver transplantation in Korea. Even though the Milan criteria have been accepted as the gold standard in deceased donor liver transplantation, the acceptable indication for living donor liver transplantation is controversial. This review covers several key issues in liver transplantation for advanced HCC: (1) recent developments and published data on expanded criteria, (2) the role of downstaging, (3) an ethical issue in expanding the criteria in living donor liver transplantation, and (4) post-operative management, including the immunosuppressive regimen and post-transplant adjuvant chemotherapy to improve survival after transplantation for advanced HCC. Biological factors, such as AFP, PIVKA-II, and a PET scan, in addition to tumor size and number, may be helpful in selecting eligible patients for liver transplantation among patients with advanced HCC. Low-level immunosuppression with low exposure of calcineurin inhibitor may reduce HCC recurrence after transplantation.
References
1). Ministry for Health, Welfare and Family Affairs. Annual Report of cancer incidence (2007), cancer prevalence (2007) and survival (1993–2007) in Korea [Internet]. Goyang-si: National Cancer Center;2009. Available from:. http://www.ncc.re.kr/manage/manage03_033_view.jsp?bbsnum=150.
2). Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–9.

3). Korean Liver Cancer Study Group, National Cancer Center. Practice guidelines for management of hepatocellular carcinoma 2009. Goyang-si: National Cancer Center;2009. Korean J Hepatol. 2009;. 15:p. 391–423. (대한간암연구회, 국립암센터. 2009 간세포암종 진료 가이드라인. 대한간학회지 2009;15: 391–423.).
4). Suh KS, Yi NJ. Liver transplantation for hepatocellular carcinoma. Korean J Hepatol. 2006; 12:493–506. (서경석, 이남준. 간세포암종 환자의 간이식. 대한간학회지 2006;12: 493–506.).
5). Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008; 8:1982–9.

6). Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002; 8:765–74.

7). Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.

8). Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007; 25:310–2.

9). Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008; 14:935–45.

10). Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008; 85:1726–32.

11). Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004; 239:150–9.

12). Marshall AE, Rushbrook SM, Vowler SL, Palmer CR, Davies RJ, Gibbs P, et al. Tumor recurrence following liver transplantation for hepatocellular carcinoma: role of tumor proliferation status. Liver Transpl. 2010; 16:279–88.

13). Kwon CH, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis. 2007; 25:313–9.

14). Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007; 13:1637–44.

15). Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Aishima S, Terashi T, et al. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation. 2007; 83:893–9.

16). Suh KS, Cho EH, Lee HW, Shin WY, Yi NJ, Lee KU. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis. 2007; 25:329–33.

17). Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006; 12:1655–60.
18). Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. A revised scoring system utilizing serum alphafeto-protein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery. 2007; 141:598–609.

19). Kornberg A, Freesmeyer M, Barthel E, Jandt K, Katenkamp K, Steenbeck J, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009; 9:592–600.

20). Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009; 50:682–7.

22). Yao FY, Kerlan RK Jr, Hirose R, Davern TJ 3rd, Bass NM, Feng S, et al. Excellent outcome following downstaging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008; 48:819–27.

23). Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002; 235:533–9.

24). Barakat O, Wood RP, Ozaki CF, Ankoma-Sey V, Galati J, Skolkin M, et al. Morphological features of advanced hepatocellular carcinoma as a predictor of downstaging and liver transplantation: an intention-to-treat analysis. Liver Transpl. 2010; 16:289–99.

25). Lo CM, Fan ST, Liu CL, Chan SC, Wong J. The role and limitation of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2004; 10:440–7.

26). Volk ML, Marrero JA, Lok AS, Ubel PA. Who decides? Living donor liver transplantation for advanced hepatocellular carcinoma. Transplantation. 2006; 82:1136–9.

27). Bruix J, Fuster J, Llovet JM. Liver transplantation for hepatocellular carcinoma: foucault pendulum versus evidence-based decision. Liver Transpl. 2003; 9:700–2.

28). Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, et al. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999; 17:324–31.

29). Kornberg A, Kupper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010; 36:275–80.

30). Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004; 10:534–40.

31). Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol [in press 2010 Mar 5].
32). Majno P, Giostra E, Mentha G. Geneva Liver Cancer Study. Is there a customised immunosuppressive regimen for patients transplanted with hepatocellular carcinoma? J Hepatol. 2005; 43:577–84.

33). Chen ZS, He F, Zeng FJ, Jiang JP, Du DF, Liu B. Early steroid withdrawal after liver transplantation for hepatocellular carcinoma. World J Gastroenterol. 2007; 13:5273–6.

34). Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anticancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004; 77:1777–82.

35). Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005; 11:497–503.

36). Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: re-assessment of risk factors for tumor recurrence. Ann Surg. 2008; 248:857–62.
37). Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the longterm risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005; 5:2954–60.

38). Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, et al. Enhanced sensitivity of PTEN-defi-cient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001; 98:10314–9.

39). Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004; 113:1774–83.

40). Toso C, Meeberg GA, Bigam DL, Oberholzer J, Shapiro AM, Gutfreund K, et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: longterm outcomes and side effects. Transplantation. 2007; 83:1162–8.

41). Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008; 14:633–8.

42). Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009; 15:1834–42.

43). Vivarelli M, Dazzi A, Zanello M, Cucchetti A, Cescon M, Ravaioli M, et al. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation. 2010; 89:227–31.

Fig. 1.
The HCC ‘Metro Ticket' – the further the distance, the higher the price. Abbreviations: HCC, hepatocellular carcinoma; UCSF, University of California San Francisco. Reprinted from Fig. 1 of reference [5].
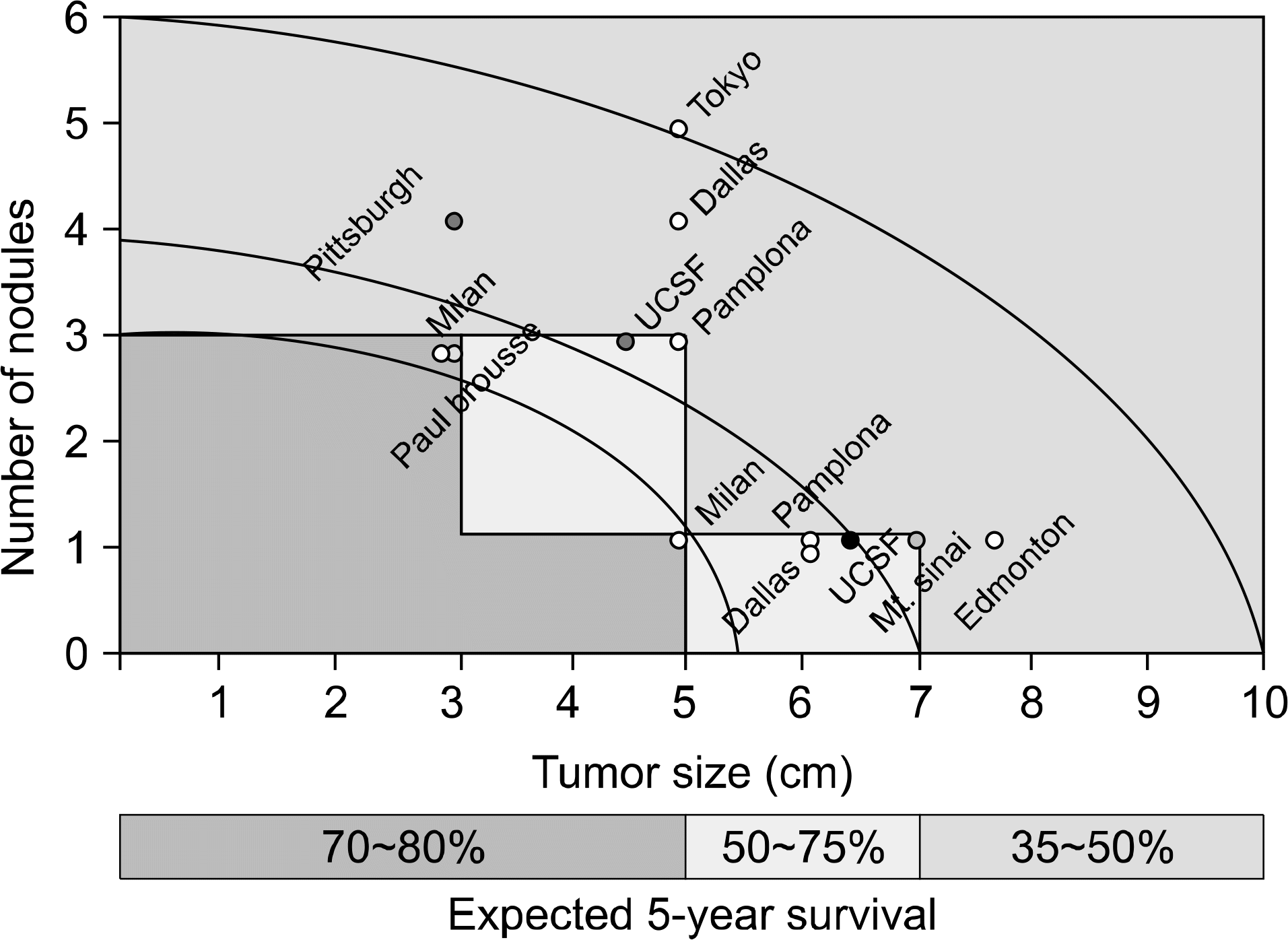
Fig. 2.
Proposed extended criteria in Asia to show comparable outcome to the Milan criteria. Abbreviations: AMC, Asan Medical Center; SMC, Samsung Medical Center; SNU, Seoul National University; UCSF, University of California San Francisco.
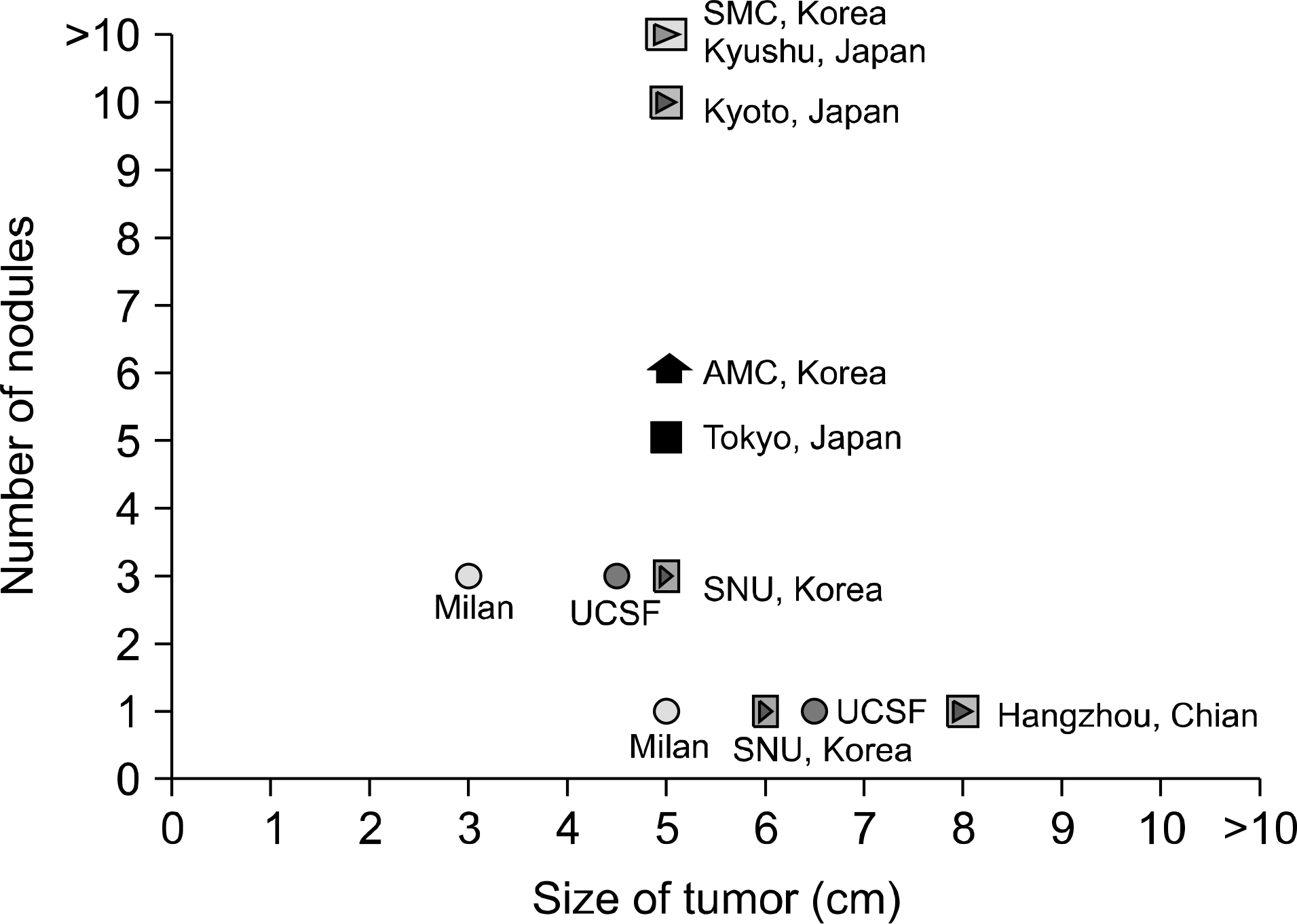
Fig. 3.
Kaplan-Meier survival analysis according to 18F-FDG PET findings. Patients with TSUVmax/LSUVmax less than 1.15 showed significantly better survival than those with TSUVmax/LSUVmax of 1.15 or more (P<0.001). Abbreviations: 18F-FDG PET, 18 fluorodeoxyglucose positron emission tomography; LSUVmax, normal liver standard uptake value max; TSUVmax, tumor standard uptake value max. Reprinted from Fig. 4 of reference [20].
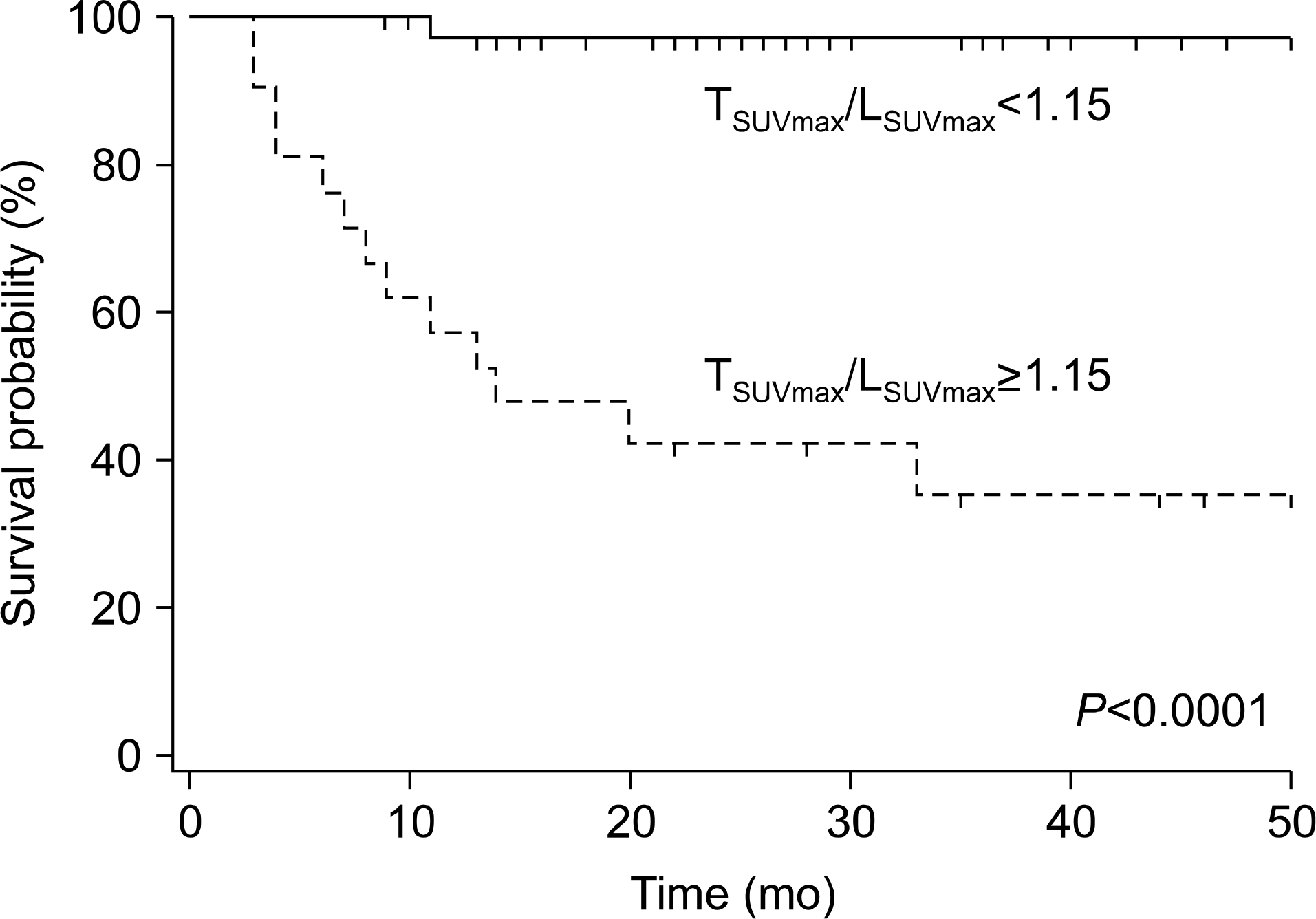
Fig. 4.
Reported activities of calcineurin inhibitors related to cancer and transplantation. Predicted effect in the presence of calcineurin inhibitors (circled arrows). Abbreviations: EBV, Epstein-Barr virus; IL-6, interleukin 6; MDR, multidrug resistance; TGF-ß, transforming growth factor; VEGF, vascular endothelial growth factor. Reprinted from Fig. 1 of reference [34].
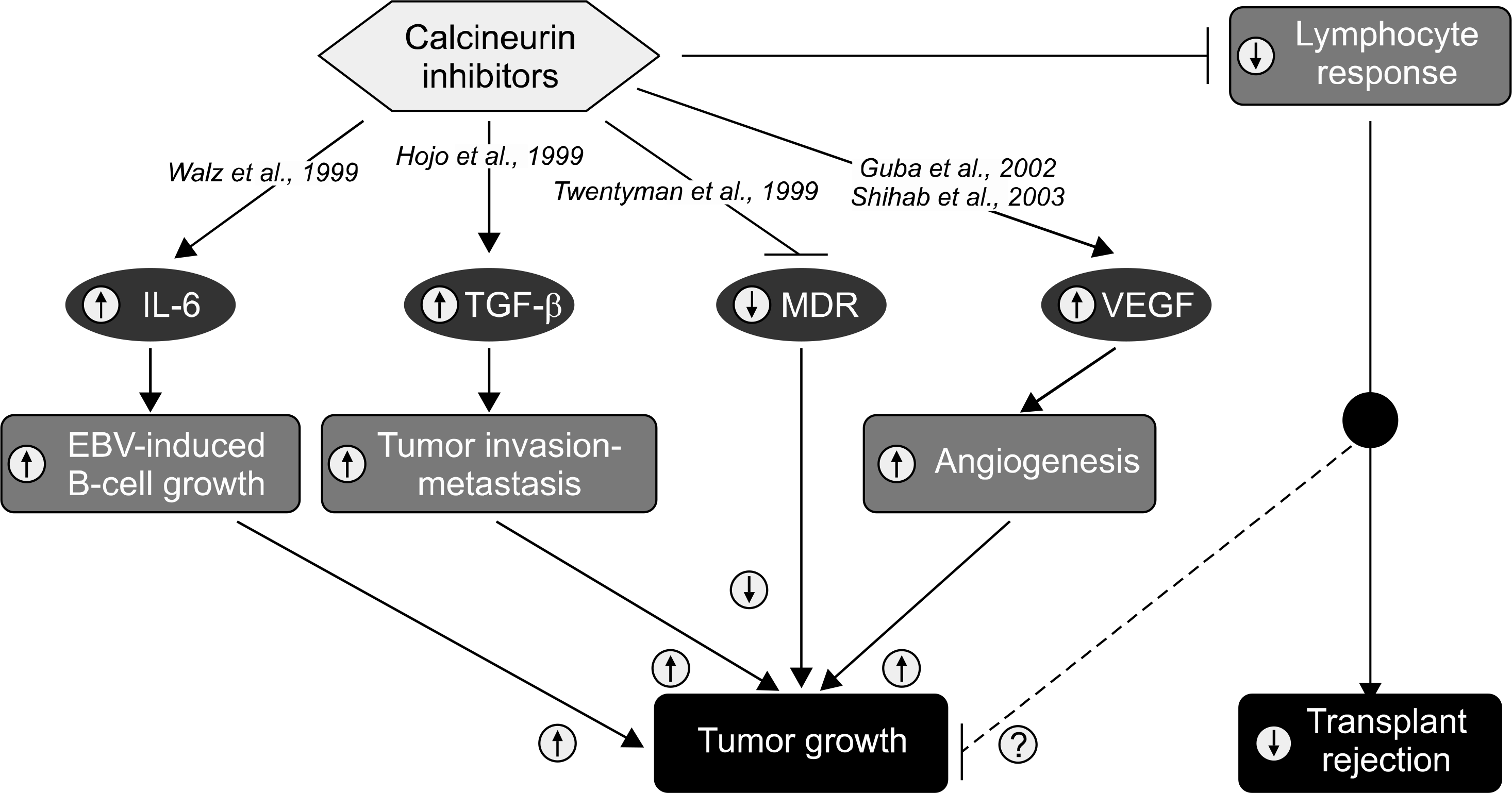
Fig. 5.
Reported activities of rapamycin related to cancer and transplantation. Predicted effect in the presence of rapamycin (circled arrows). Abbreviations: IL-6, interleukin 6; mTOR, mammalian target of rapamycin; PTEN, phosphatase and tension homologue deleted on chromosome 10; VEGF, vascular endothelial growth factor. Reprinted from Fig. 2 of reference [34].
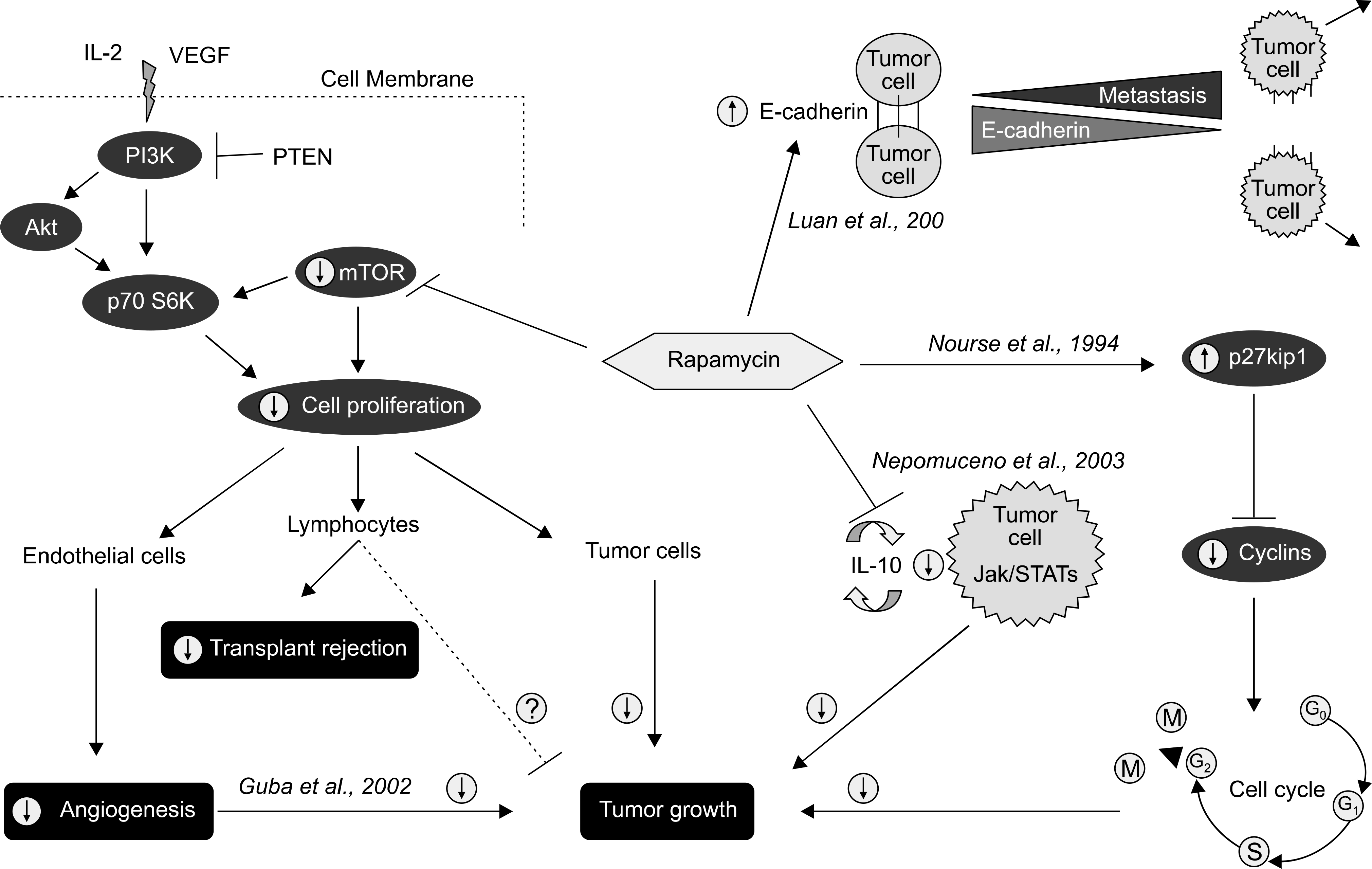
Table 1.
Expanded criteria in Asian centers
Table 2.
Reports on mTOR inhibitors to reduce HCC recurrence after liver transplantation for hepatocellular carcinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download