Abstract
Background
Chronic allograft nephropathy (CAN), which causes graft failure, is related to tubular atrophy and interstitial fibrosis. E-cadherin is a well-known epithelial marker and heat shock protein (HSP)-47 is a collagen-specific molecular chaperone that regulates collagen synthesis. Transforming growth factor (TGF)-β1, a profibrotic cytokine, downregulates E-cadherin and induces expression of mesenchymal markers in an in vitro model. C4d expression is considered a poor prognostic marker for graft survival. This study evaluated the relationship between the expression of E-cadherin, HSP47, TGF-β1, and C4d with the prognosis for CAN.
Methods
Between March 1991 and August 2007, we performed renal allograft biopsies on 42 recipients with deteriorating renal function. CAN was diagnosed according to the chronic allograft damage index (Banff classification). Renal allograft biopsies were examined for the expression of E-cadherin, HSP47, TGF-β1, or C4d by immunohistochemistry. The HSP47, TGF-β1, and E-cadherin staining was scored semiquantitatively by analyzing ten different fields of cortical interstitium and tubules. Biopsies with endothelial C4d staining in peritubular capillaries (≥25%) were designated as C4d-positive.
Results
Of 42 recipients, 17 (40.5%) were in the graft survival group (GS) and 25 (59.5%) were in the graft failure group (GF). E-cadherin expression in tubular cells of the GS was much higher than that of the GF (94.1% vs 52%, P=0.04). HSP47 expression in tubular cells and interstitium in the GF was much higher than that in the GS (84% vs 35.3%, P=0.001). TGF-β1 expression in tubular cells and interstitium in the GF was much higher than that in the GS (72% vs 23.5%, P=0.02).
Go to : 
References
1). United States Renal Data System. USRDS 2003 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD.: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases;2003. p. 1–560.
2). In Danovitch GM. Handbook of kidney transplantation. 4th ed.Philadelphia: Lippincott Williams & Wilkins;2005. : 242.
3). Kreis HA, Ponticelli C. Causes of late renal allograft loss: chronic allograft dysfunction, death, and other factors. Transplantation. 2001; 71(11 Suppl):SS5–9.
4). Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells infibrogenesis. Am J Transplant. 2005; 5:1367–74.
5). Ohba K, Miyata Y, Koga S, Nishikido M, Kanetake H, Nazneen A, et al. Interstitial expression of heat-shock protein 47 correlates with capillary deposition of complement split product C4d in chronic allograft nephropathy. Clin Transplant. 2005; 19:810–6.

6). Razzaque MS, Kumatori A, Harada T, Taguchi T. Coexpression of collagens and collagen-binding heat shock protein 47 in human diabetic nephropathy and IgA nephropathy. Nephron. 1998; 80:434–43.

7). Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005; 24:5764–74.
8). Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002; 13:2371–80.

9). Solez K, Vincenti F, Filo RS. Histopathologic findings from 2-year protocol biopsies from a U.S. multicenter kidney transplant trial comparing tacrolimus versus cyclosporine: a report of the FK506 Kidney Transplant Study Group. Transplantation. 1998; 66:1736–40.
10). Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–23.

11). Halloran PF. Call for revolution: a new approach to describing allograft deterioration. Am J Transplant. 2002; 2:195–200.

12). Freese P, Svalander CT, Molne J, Norden G, Nyberg G. Chronic allograft nephropathy-biopsy finding and outcome. Nephrol Dial Transplant. 2001; 16:2401–6.
13). Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002; 110:341–50.

14). Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004; 15:1–12.

15). Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological and experimentally induced. Am J Kidney Dis. 1995; 26:678–90.

16). Birchmeier W, Birchmeier C. Epithelial-mesenchymal transitions in development and tumor progression. EXS. 1995; 74:1–15.

17). Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002; 62:137–46.

18). Razzaque MS, Le VT, Taguchi T. Heat shock protein 47 and renal fibrogenesis. Contrib Nephrol. 2005; 148:57–69.

19). Abe K, Ozono Y, Miyazaki M, Koji T, Shioshita K, Furusu A, et al. Interstitial expression of heat shock protein 47 and alpha-smooth muscle actin in renal allograft failure. Nephrol Dial Transplant. 2000; 15:529–35.
20). Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, et al. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001; 159:1313–21.
21). Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and an-giotensin-converting enzyme inhibition: a patient-level metaanalysis. Ann Intern Med. 2003; 139:244–52.

22). Bijian K, Cybulsky AV. Stress proteins in glomerular epithelial cell injury. Contrib Nephrol. 2005; 148:8–20.

23). Zoja C, Corna D, Camozzi D, Cattaneo D, Rottoli D, Batani C, et al. How to fully protect the kidney in a severe model of progressive nephropathy: a multidrug approach. J Am Soc Nephrol. 2002; 13:2898–908.

24). Suthanthiran M, Khanna A, Cukran D, Adhikarla R, Sharma VK, Singh T, et al. Transforming growth factor-beta 1 hyperexpression in African American end-stage renal disease patients. Kidney Int. 1998; 53:639–44.
25). Park MG, Joo SH, Park SK, Kim JS, Hyun SJ, Nam ES, et al. Correlation of hypoxia inducible factor-1α & transforming grwth factor-1β expression and progression of renal allograft. J Korean Soc Transplant. 2005; 19:131–6. (박민근, 주선형, 박성길, 김주섭, 현숙자, 남은숙, 등. 신생검에서의 Hypoxia Inducible Factor-1α, Transforming Growth Factor-β1 발현과 이식신 예후와의 연관성. 대한이식학회지 2005;19: 131–6.).
26). Lorenz M, Regele H, Schillinger M, Exner M, Rasoul Rokenschaub S, Wahrmann M, et al. Risk factors for capillary C4d deposition in kidney allografts: evaluation of a large study cohort. Transplantation. 2004; 78:447–52.

27). Bohmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002; 13:1091–9.
Go to : 
 | Fig. 1.E-cadherin expression in Implantation biopsy vs Functioning group (CAN) vs non functioning group (CAN), arrow (high expression), arrow head (low expression) (×100). (A) Implantation biopsy, high expression of E-cadherin. (B) CAN, Functioning group mild loss of expression of E-cadherin. (C) CAN, non functioning group entire loss of expression. Abbreviation: CAN, chronic allograft nephropathy. |
 | Fig. 2.HSP47 expression in Implantation biopsy vs Functioning group (CAN) vs non functioning group (CAN), arrow (high expression), arrow head (low expression) (×100). (A) Implantation biopsy, no expression of HSP47. (B) CAN, functioning group, low expression in tubule. (C) CAN, non functioning group, high expression in tubule and interstitium. Abbreviations: HSP, heat shock protein; CAN, chronic allograft nephropathy. |
 | Fig. 3.TGF-ß1 expression in implantation biopsy vs functioning group (CAN) vs non functioning group (CAN), arrow (high expression), arrow head (low expression) (×100). (A) Implantation biopsy, no expression of TGF-ß1. (B) CAN, Functioning group mild expression in interstitium. (C) CAN, non functioning group high expression in tubule & inerstitium. Abbreviations: TGF, transforming growth factor; CAN, chronic allograft nephropathy. |
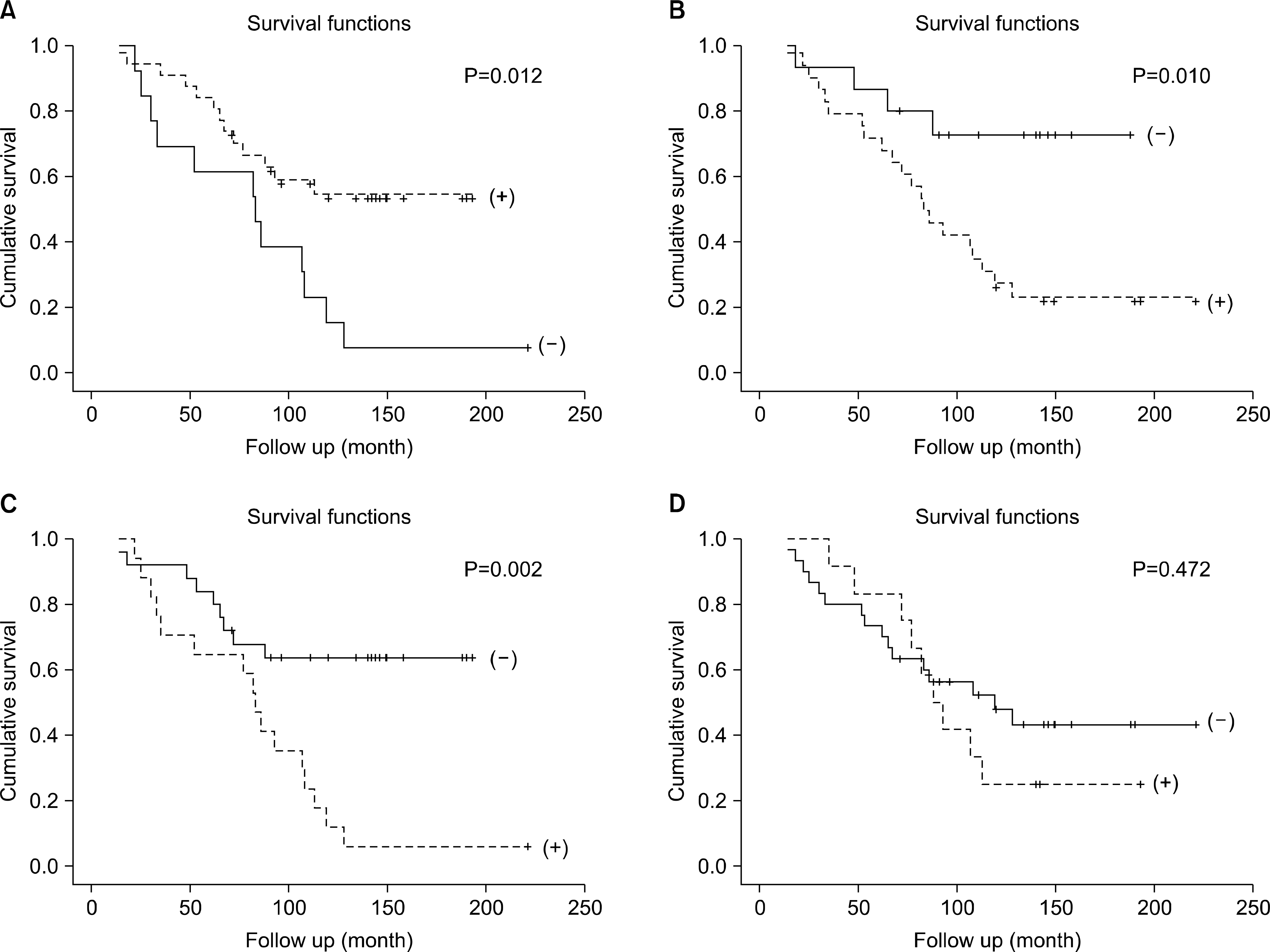 | Fig. 4.Correlation of E-cadherin E-cadherin, HSP-47, TGF-β1, C4d expression and graft survivals after transplantation. (A) E-cadherin.(B) HSP-47. (C) TGF-b1. (D) C4d. Abbreviations: HSP, heat shock protein; TGF, transforming growth factor. |
Table 1.
Banff 97 diagnostic categories for renal allograft biopsies-update
Table 2.
Patients characteristics in post-transplant chronic allograft nephropathy
Table 3.
Correlation between Banff score and functioning graft/ graft loss
Table 4.
Correlation between markers and proteinuria, serum creatinine
| Marker | Proteinuria | P | Cr a | P | ||
|---|---|---|---|---|---|---|
| (+) | (−) | |||||
| E-cadherin | (+) | 11 | 2 | 0.96 | 2.75 | 0.40 |
| (−) | 20 | 9 | 3.04 | |||
| HSP47 | (+) | 11 | 16 | 0.019 | 3.05 | 0.477 |
| (−) | 1 | 14 | 2.46 | |||
| TGF-ß1 | (+) | 5 | 20 | 0.136 | 3.10 | 0.380 |
| (−) | 7 | 10 | 2.40 | |||
| C4d | (+) | 3 | 9 | 0.746 | 4.14 | <0.05 |
| (−) | 9 | 21 | 2.37 | |||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download