Abstract
Background
Novel H1N1 influenza A was a pandemic disease in 2009. However, limited data are available on renal transplant recipients undergoing long-term immunosuppression who contracted novel H1N1 influenza A.
Methods
We analyzed 2,345 patients who had been tested with H1N1 swab real-time reverse transcriptase-polymerase chain reaction test (rRT-PCR) between May 2009 and February 2010. Of them, 30 were kidney recipients who underwent kidney transplantation between April 1979 and 2, May 2009 before the first diagnosis of H1N1 influenza A in Korea. The clinical characteristics, treatment, and outcome of renal transplant recipients with confirmed H1N1 influenza were reviewed retrospectively.
Results
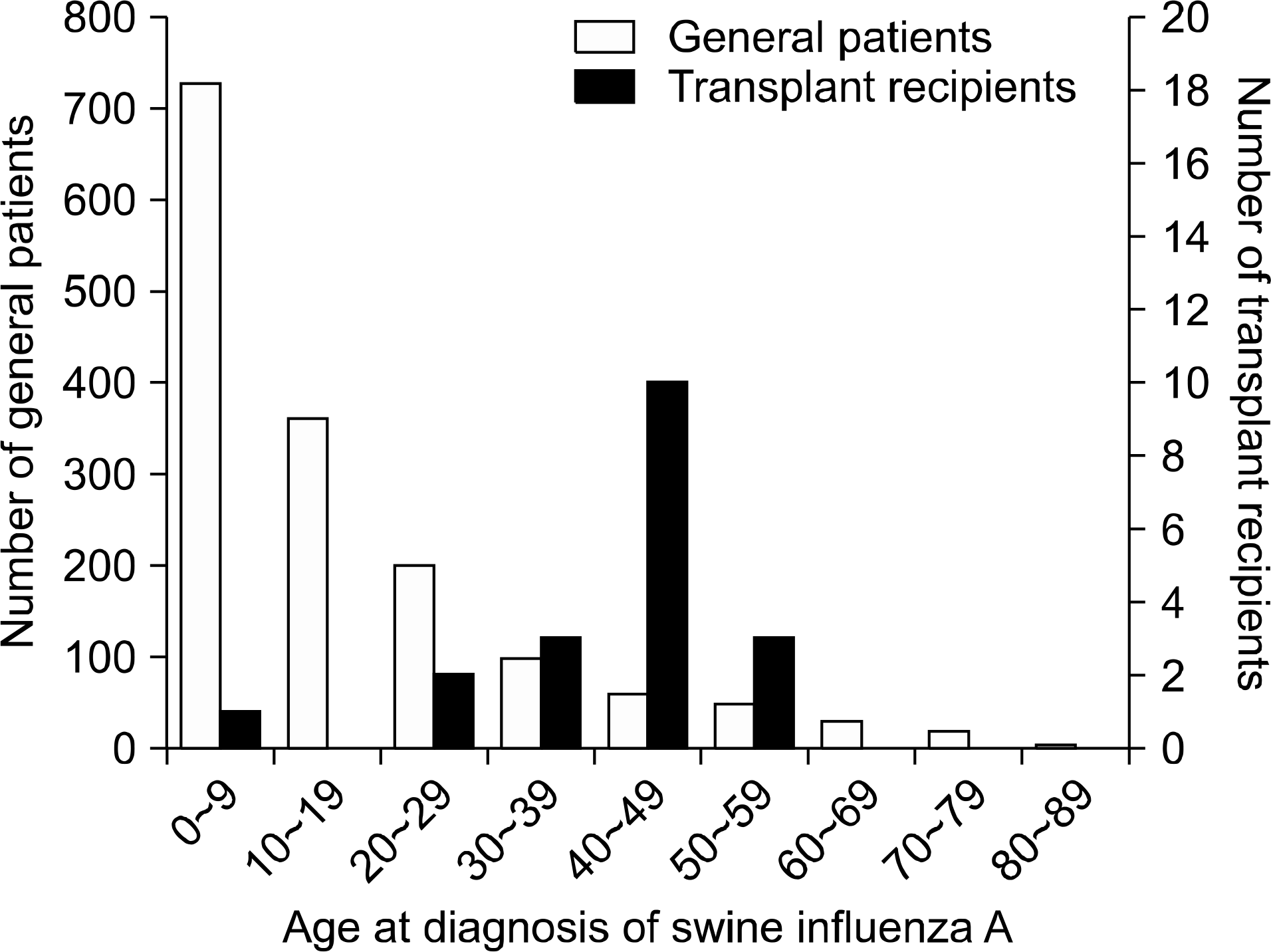
A total of 1,543 (66.7%) general patients were swine influenza A confirmed. Of the 30 transplant patients, 19 (63.3%) were confirmed with swine influenza A. The mean age of the general patients at diagnosis of swine influenza A was younger than that of renal recipients (16.5±16.1 vs. 39.7±11.5 years, P<0.0001). More patients died in the transplant group than in the general patient group even after oseltamivir (Tamiflu) treatment. When comparing the cured group with the dead group of transplant patients, the dead group had a longer duration between symptom manifestation and the beginning of treatment than the cured group (7 [5–7] vs. 2 [1–14] days, P=0.007). The dead group presented more complications such as pneumonia (P=0.009).
Go to : 
References
1). World Health Organization (WHO). Pandemic (H1N1) 2009 update 110 [Internet]. Geneva, Switzerland: WHO;2009. Available from:. http://www.who.int/csr/don/2010_07_23a/en/index.html.
2). Korea Centers for Disease Control and Prevention (KCDC). Influenza surveillance and laboratory monitoring results 2009–2010 [Internet]. Seoul: KCDC;2010. Available from:. http://flu.cdc.go.kr. (질병관리본부. 인플루엔자 표본감시 및 실험실 감시 결과 [Internet]. 서울: 질병관리본부; 2010. Available from:. http://flu.cdc.go.kr. .).
4). Sym D, Patel PN, El-Chaar GM. Seasonal, avian, and novel H1N1 influenza: prevention and treatment modalities. Ann Pharmacother. 2009; 43:2001–11.

5). Zapata R, Uribe M, Martinez W, Andrade A, Leal JL, Gomez F. Severe novel H1N1 influenza A infection in the immediate postoperative period of a liver transplant patient. Liver Transpl. 2010; 16:447–52.

6). Vilchez RA, McCurry K, Dauber J, Lacono A, Griffith B, Fung J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002; 2:287–91.

7). Division of Public Health Crisis Response, Center for Communicable Disease Surveillance and Response, Korea Centers for Disease Control and Prevention (KCDC). Korean countermeasure and plan against influenza A (H1N1) infection in humans. Public Health Wkly Rep. 2009; 2:279–81. (질병관리본부 전염병대응센터 공중보건위기대응과. 국내 신종인플루엔자 A (H1N1) 인체감염에 대한 국가위기대응 및 계획. 주간건강과 질병 2009;2: 279–81.).
8). Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2009 H1N1 influenza. Mayo Clin Proc. 2010; 85:64–76.

9). Temte JL. Basic rules of influenza: how to combat the H1N1 influenza (swine flu) virus. Am Fam Physician. 2009; 79:938–9.
10). Jeannot AC, Hamoudi M, Bourayou N, Tabuteau C, Garandeau C, Trapateau JM, et al. First cases of secondary transmission of a novel swine-origin influenza A (H1N1) virus in France. Med Mal Infect. 2010; 40:48–50.
11). Lye DC, Chow A, Tan A. Oseltamivir therapy and viral shedding in pandemic (H1N1) 2009. In: Program and Abstracts of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco, California, USA. Washington DC: American Society for Microbiology;. 2009. : abstract V-1269c.
12). Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J Infect Dis. 2006; 193:760–4.

13). Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003; 348:867–8.

14). Kumar D, Morris MI, Kotton CN, Fischer SA, Michaels MG, Allen U, et al. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant. 2010; 10:18–25.

15). Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009; 361:674–9.

16). Aschan J, Ringdén O, Ljungman P, Andersson J, Lewensohn-Fuchs I, Forsgren M. Influenza B in transplant patients. Scand J Infect Dis. 1989; 21:349–50.

17). Ljungman P, Gleaves CA, Meyers JD. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989; 4:35–40.
18). Centers for Disease Control and Prevention (CDC). Swine-origin influenza A (H1N1) virus infections in a school – New York City, April 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:470–2.
19). Department of Health. Pandemic H1N1 2009 influenza: clinical management guidelines for adults and children [Internet]. London, England: Department of Health;2009. Available from:. http://www.dh.gov.uk/prod_con-sum_dh/groups/dh_digitalassets/@dh/@en/@ps/@sta/@perf/documents/digitalasset/dh_110617.pdf.
20). World Health Organization (WHO). Clinical management of human infection with pandemic (H1N1) 2009: revised guidance [Internet]. Geneva, Switzerland: WHO;2009. Available from:. http://www.who.int/csr/resources/publications/swineflu/clinical_management_h1n1.pdf.
21). Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009; 361:1935–44.

22). Denholm JT, Gordon CL, Johnson PD, Hewagama SS, Stuart RL, Aboltins C, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust. 2010; 192:84–6.

23). de Sandes Freitas TV, Ono G, Correa L, Gomes PS, Tedesco Silva H, Carmargo LF, et al. Clinical features and outcome of H1N1 influenza A infection among kidney transplant recipients. Am J Transplant. 2010; 10(Suppl 4):127.
24). Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44(Suppl 2):S27–72.

25). Keane WR, Helderman JH, Luby J, Gailiunas P, Hull AR, Kokko JP. Epidemic renal transplant rejection associated with influenza A victoria. Proc Clin Dial Transplant Forum. 1978; 8:232–6.
26). Smith KG, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transplant. 1998; 13:160–4.

27). Versluis DJ, Beyer WE, Masurel N, Wenting GJ, Weimar W. Impairment of the immune response to influenza vaccination in renal transplant recipients by cyclosporine, but not azathioprine. Transplantation. 1986; 42:376–9.

28). Keshtkar-Jahromi M, Argani H, Rahnavardi M, Mirchi E, Atabak S, Tara SA, et al. Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: a controlled trial. Am J Nephrol. 2008; 28:654–60.

29). Khanna N, Steffen I, Studt JD, Schreiber A, Lehmann T, Weisser M, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2009; 11:100–5.

30). Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004; 39:1300–6.

31). Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000; 355:1845–50.
32). Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, et al. Early administration of oral oseltamivir increses the benefits of influenza treatment. J Antimicrob Chemother. 2003; 51:123–9.
33). Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009; 361:680–9.

34). Klimov AI, Rocha E, Hayden FG, Shult PA, Roumillat LF, Cox NJ. Prolonged shedding of amantadine-resistant influenzae A viruses by immunodeficient patients: detection by polymerase chain reaction-restriction analysis. J Infect Dis. 1995; 172:1352–5.
35). Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients-Seatt, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:893–6.
Go to : 
Table 1.
Comparison of clinical characteristics in general versus transplant patients
Table 2.
Clinical characteristics of three transplant groups
| Factors | Group A a | Group B b | Group C c | P |
|---|---|---|---|---|
| Sex | 0.121 | |||
| Male | 1,253 | 5 | 9 | |
| Female | 695 | 6 | 10 | |
| Age at transplantation | 38 (2∼71) | 27 (15∼51) | 44 (9∼54) | 0.078 |
| Main immunosuppressive agent | 0.784 | |||
| Azathioprine | 41 (2.1%) | 0 (0.0%) | 0 (0.0%) | |
| Cyclosporine A | 1,433 (73.6%) | 7 (63.6%) | 14 (73.7%) | |
| Tacrolimus | 474 (24.3%) | 4 (36.4%) | 5 (26.3%) | |
| Antimetabolite | 0.918 | |||
| Yes | 1,035 (53.1%) | 6 (54.5%) | 9 (47.4%) | |
| No | 913 (46.9%) | 5 (45.5%) | 10 (53.6%) | |
| Retransplantation | 0.739 | |||
| Yes | 159 (8.2%) | 0 (0.0%) | 2 (10.5%) | |
| No | 1,789 (91.8%) | 11 (100%) | 17 (89.5%) | |
| Acute rejection episode | 0.433 | |||
| Yes | 469 (24.1%) | 1 (9.1%) | 6 (31.6%) | |
| No | 1,479 (75.9%) | 10 (90.9%) | 13 (68.4%) |
Table 3.
Factors that affect to the treatment outcome in transplant patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download