Abstract
Background:
Severe graft dysfunction has been occasionally encountered following adult living donor liver transplantation (LDLT). This study intended to assess the effectiveness of plasmapheresis (PP) as a liver supportive measure in LDLT recipients showing severe graft dysfunction.
Methods:
During 1 year of 2007, 276 adult LDLTs were performed in our institution. Of them 27 underwent PP therapy as a liver support.
Results:
Seventeen underwent PP during the first month following LDLT and another 10 underwent PP after that period. The underlying causes of such liver support were acute and chronic rejections, ischemic damage, viral hepatitis recurrence and unknown causes. A total of 329 sessions of PP were performed for these 27 patients, indicating 12.2±9.9 times per patient for 28.1±32.2 days. Concurrent hemodiafiltration was done in 66.7%. Serum total bilirubin level was significantly reduced following PP therapy: 23.2±6.5 mg/dL before PP and 14.4±5.6 mg/dL at 1 week after completion of PP (P<0.001). Other biochemical parameters did not significantly affected by PP. Overall 1-year patient survival rate was 63.0%. Six-month graft survival rate after completion of PP was 82.6% in 17 patients undergoing PP during the first posttransplant month and 30% in 10 patients undergoing PP after 1 month (P= 0.013).
Conclusions:
The results of this study implicate that PP has a beneficial effect on the recovery of liver graft function, especially during the early posttransplant period. We suggest to perform active application of PP therapy for liver recipients showing severe graft dysfunction of total bilirubin greater than 15∼20 mg/dL.
Go to : 
REFERENCES
1). Naruse K, Nagashima H, Sakai Y, Kokudo N, Makuuchi M. Development and perspectives of perfusion treatment for liver failure. Surg Today. 2005; 35:507–17.

2). Chiu A, Chan LM, Fan ST. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int. 2006; 26:695–702.

3). Gaspari R, Avolio AW, Zileri Dal Verme L, Agnes S, Proietti R, Castagneto M, et al. Molecular adsorbent recirculating system in liver transplantation: safety and efficacy. Transplant Proc. 2006; 38:3544–51.

4). Ozdemir FN, Tutal E, Sezer S, Gür G, Bilgic A, Haberal M. Effect of supportive extracorporeal treatment in liver transplantation recipients and advanced liver failure patients. Hemodial Int. 2006; 10(S2):28–32.

5). Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, et al. Toward 300 liver transplants a year. Surg Today. 2009; 39:367–73.

6). Schmidt LE, Wang LP, Hansen BA, Larsen FS. Systemic hemodynamic effects of treatment with the molecular ad-sorbents recirculating system in patients with hyperacute liver failure: a prospective controlled trial. Liver Transpl. 2003; 9:290–7.

7). Hetz H, Faybik P, Berlakovich G, Baker A, Bacher A, Burghuber C, et al. Molecular adsorbent recirculating system in patients with early allograft dysfunction after liver transplantation: a pilot study. Liver Transpl. 2006; 12:1357–64.

8). Kellersmann R, Gassel HJ, Bühler C, Thiede A, Timmermann W. Application of Molecular Adsorbent Recirculating System in patients with severe liver failure after hepatic resection or transplantation: initial sin-gle-centre experiences. Liver. 2002; 22(S2):56–8.

9). Steiner C, Mitzner S. Experiences with MARS liver support therapy in liver failure: analysis of 176 patients of the International MARS Registry. Liver. 2002; 22(S2):20–5.
10). Gaspari R, Cavaliere F, Sollazzi L, Perilli V, Melchionda I, Agnes S, et al. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant Proc. 2009; 41:253–8.

11). Novelli G, Rossi M, Pretagostini M, Pugliese F, Ruberto F, Novelli L, et al. One hundred sixteen cases of acute liver failure treated with MARS. Transplant Proc. 2005; 37:2557–9.

12). Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, et al. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006; 21:96–9.

13). Bektas M, Idilman R, Soykan I, Soydan E, Arat M, Cinar K, et al. Adjuvant therapeutic plasma exchange in liver failure: assessments of clinical and laboratory parameters. J Clin Gastroenterol. 2008; 42:517–21.
14). Hwang S, Ha TY, Ahn CS, Kim KH, Lee SG. Reappraisal of plasmapheresis as a supportive measure in a patient with hepatic failure after major hepatectomy. Case Rep Gastroenterol. 2007; 1:162–7.

15). Buckner CD, Clift RA, Volwiler W, Donohue DM, Burnell JM, Saunders FC, et al. Plasma exchange in patients with fulminant hepatic failure. Arch Intern Med. 1973; 132:487–92.

16). Mandal AK, King KE, Humphreys SL, Maley WR, Burdick JF, Klein AS. Plasmapheresis: an effective therapy for primary allograft nonfunction after liver transplantation. Transplantation. 2000; 70:216–20.
17). Morimoto T, Matsushima M, Sowa N, Ide K, Sawanishi K. Plasma adsorption using bilirubin-adsorbent materials as a treatment for patients with hepatic failure. Artif Organs. 1989; 13:447–52.

18). Singer AL, Olthoff KM, Kim H, Rand E, Zamir G, Shaked A. Role of plasmapheresis in the management of acute hepatic failure in children. Ann Surg. 2001; 234:418–24.

19). Camci C, Akdogan M, Gurakar A, Gilcher R, Rose J, Monlux R, et al. The impact of total plasma exchange on early allograft dysfunction. Transplant Proc. 2004; 36:2567–9.

20). Hwang S, Lee SG, Jung DH, Kim KH, Ha TY, Song GW. Simulation of deceased-donor liver graft allocation as UNOS status I or IIa on the current Korean setting for patients with hepatitis B virus-induced fulminant hepatic failure. Korean J Hepatobiliary Pancreat Surg. 2009; 13:31–6.
21). Yamamoto R, Nagasawa Y, Marubashi S, Furumatsu Y, Iwatani H, Iio K, et al. Early plasma exchange for pro-gressive liver failure in recipients of adult-to-adult living-related liver transplants. Blood Purif. 2009; 28:40–6.

22). Bektas M, Idilman R, Soykan I, Soydan E, Arat M, Cinar K, et al. Adjuvant therapeutic plasma exchange in liver failure: assessments of clinical and laboratory parameters. J Clin Gastroenterol. 2008; 42:517–21.
Go to : 
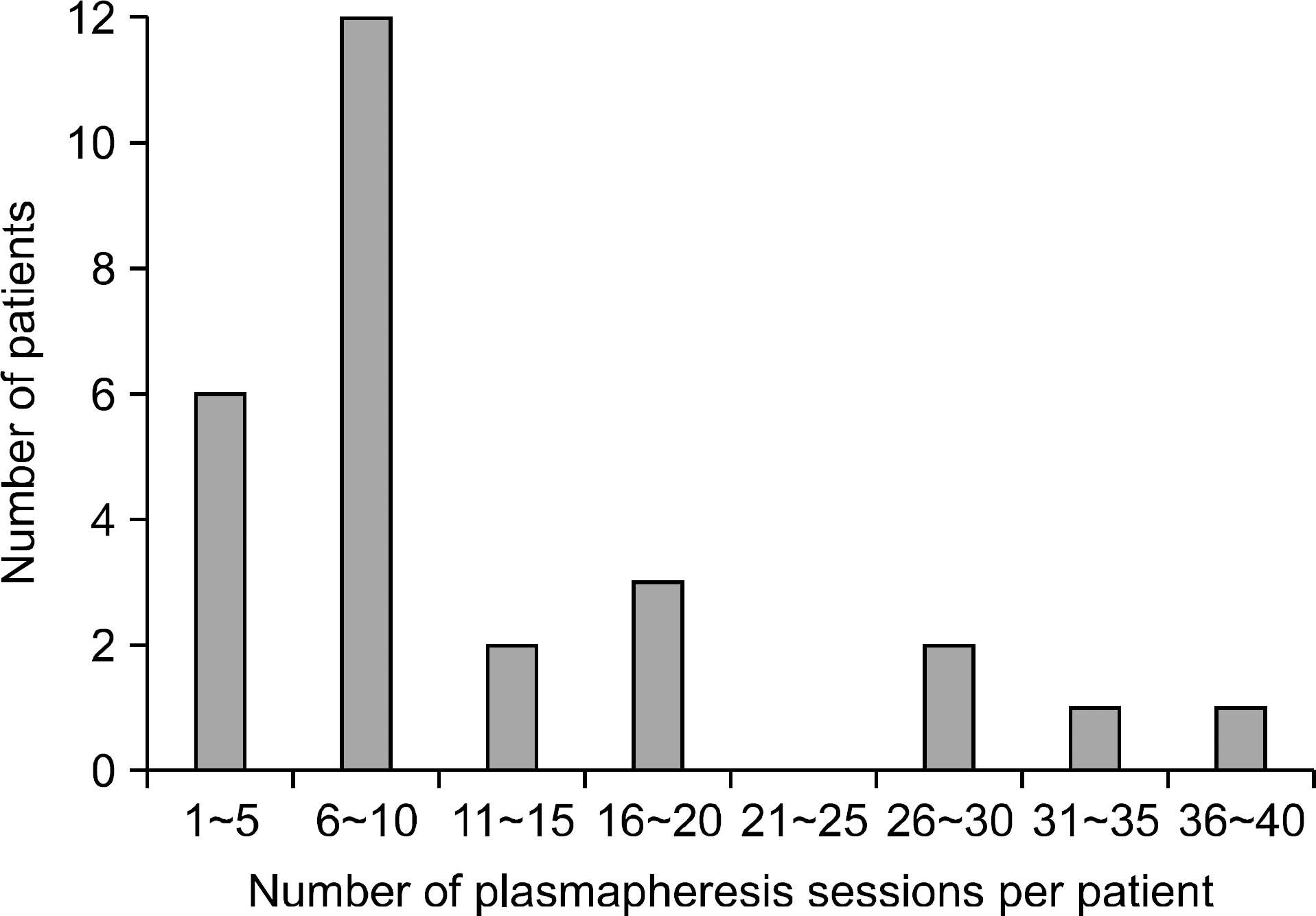 | Fig. 1.Number of plasmapheresis sessions per one recipient undergone living donor liver transplantation. |
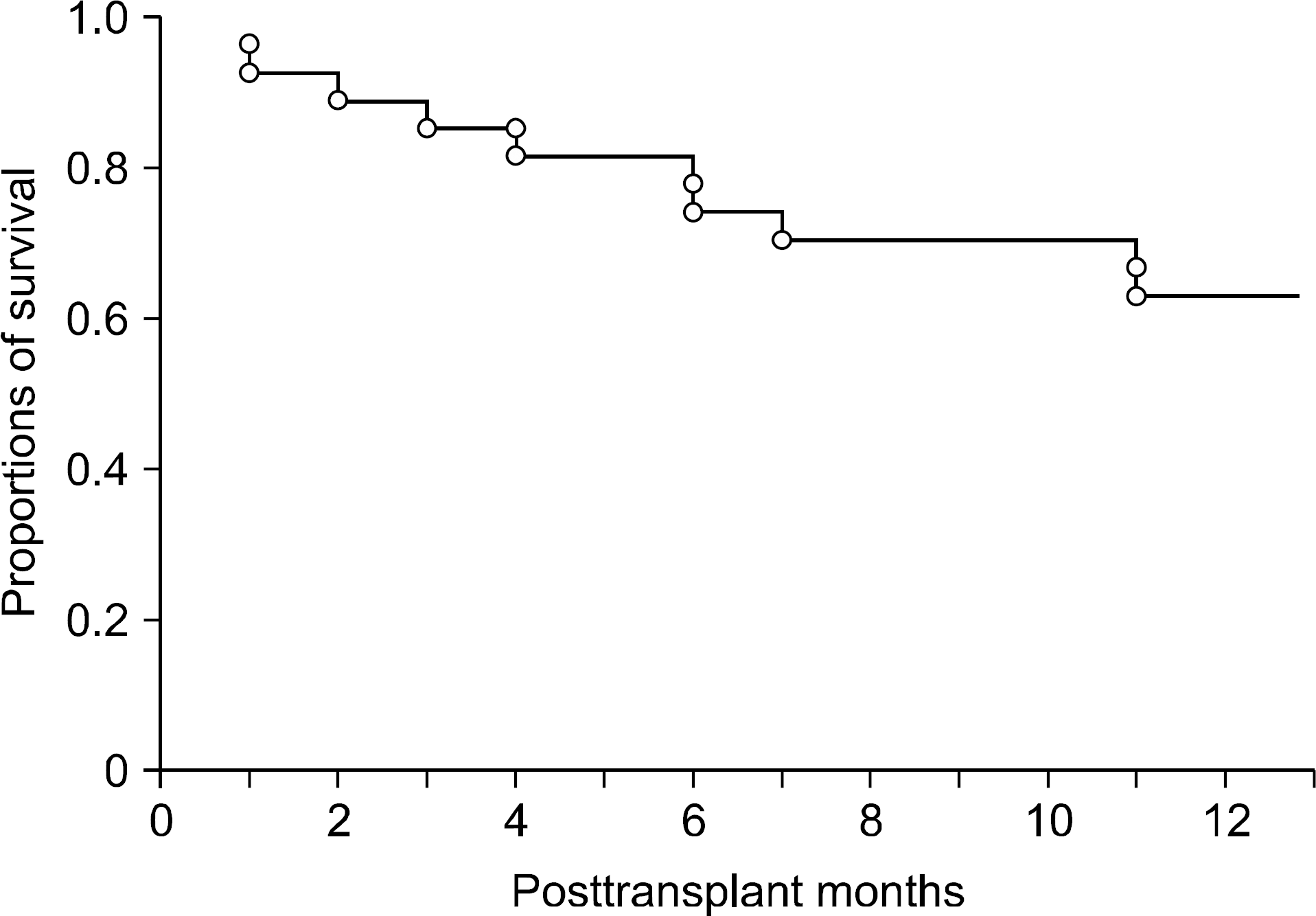 | Fig. 2.Overall patient survival curve of 27 adult living donor liver transplant recipients undergoing plasmapheresis for liver support. |
Table 1.
Comparison of the biochemical profiles in 27 adult living donor liver transplant recipients undergoing plasmapheresis (PP) for liver support
| Before start of PP | After 1 week during PP | 1 week after last PP | P-value∗ | |
|---|---|---|---|---|
| Total bilirubin (mg/dL) | 23.2±6.5 | 19.6±8.9 | 14.4±5.6 | <0.001 |
| Prothrombin time (INR) | 1.47±0.35 | 1.38±0.37 | 1.33±0.63 | 0.317 |
| AST (IU/L) | 120.3±341.7 | 145.6±212.3 | 108.4±98.5 | 0.747 |
| ALT (IU/L) | 148.4±287.1 | 156.0±198.8 | 98.2±86.4 | 0.389 |
| Ammonia (μ mol/L) | 75.6±40.1 | 75.8±41.3 | 73.2±37.8 | 0.822 |
| Lactate (μ mol/L) | 3.4±2.1 | 3.7±2.6 | 3.6±4.1 | 0.811 |
| Cholesterol (mg/dL) | 74.3±40.8 | 89.6±47.4 | 96.5±55.1 | 0.098 |
| Albumin (g/dL) | 2.9±0.5 | 3.3±0.3 | 3.2±0.8 | 0.105 |
| BUN (mg/dL) | 55.4±27.1 | 49.8±28.1 | 42.7±25.4 | 0.081 |
| Creatinine (mg/dL) | 1.67±1.1 | 1.52±0.9 | 1.38±0.7 | 0.253 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download