Abstract
Background:
A positive reaction at flow cytometry crossmatch (FCXM) has been highlighted by its predictive value for clinical outcome in kidney transplantation after accumulation of large clinical data. The detection of de novo development of anti-HLA antibodies after transplantation is associated with increased rejection and decreased graft survival. In this study, we report the experience for the detection of antidonor specific antibody (DSA) by more sensitive FCXM methods in renal transplantation patients.
Methods:
T and B cell FCXMs were performed on 11 pretransplant and 51 posttransplant sera from 11 patients who received renal grafts between 2004 and 2005. The posttransplant sera were collected in specific and regular intervals from posttranspant 1 week to 1 year.
Results:
Among 62 sera, four (7.8%) from 2 patients showed positive FCXM. In one patient, pretransplant serum which was negative at previous CDCXM, and 2 consecutive sera collected at 1 week and 1 month after transplantation were positive at FCXM. And the antibody identified was B51 which was specific for one of donor alleles (DSA). In another patient, FCXM became positive 1 week after transplantation although pretransplant serum had negative results at both CDCXM and FCXM. Both patients had experienced more than one rejection episodes.
Conclusions:
Detection of DSA with more sensitive technique such as flow cytometry based method clearly displayed a beneficial effect for prediction of clinical outcome as a part of pretransplant compatibility test, and also as a posttransplant monitoring test to identify the de novo production of clinically significant DSA.
Go to : 
REFERENCES
1). McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000; 69:319–26.
2). Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966; 2:662–5.

3). Scornik JC, Bray RA, Pollack MS, Cook DJ, Marrari M, Duquesnoy R, et al. Multicenter evaluation of the flow cytometry T-cell crossmatch: results from the American Society of Histocompatibility and Immunogenetics- College of American Pathologists proficiency testing program. Transplantation. 1997; 63:1440–5.
4). Scornik JC, Clapp W, Patton PR, Van der Werf WJ, Hemming AW, Reed AI, et al. Outcome of kidney transplants in patients known to be flow cytometry crossmatch positive. Transplantation. 2001; 71:1098–102.
5). Rebibou JM, Bittencourt MC, Saint-Hillier Y, Chabod J, Dupont I, Bittard H, et al. T-cell flow-cytometry crossmatch and long-term renal graft survival. Clin Transplant. 2004; 18:558–63.

6). Rodríguez PC, Palacio JL, Arango L, Henao JE, García LF. Detection of allo-and autoantibodies in kidney transplantation by flow cytometry. Transplant Proc. 1999; 31:282–4.
7). Wahrmann M, Exner M, Schillinger M, Haidbauer B, Regele H, Körmöczi GF, et al. Pivotal role of complement-fixing HLA alloantibodies in presensitized kidney allograft recipients. Am J Transplant. 2006; 6:1033–41.

8). El Fettouh HA, Cook DJ, Bishay E, Flechner S, Goldfarb D, Modlin C, et al. Association between a positive flow cytometry crossmatch and the development of chronic rejection in primary renal transplantation. Urology. 2000; 56:369–72.

9). O'Rourke RW, Osorio RW, Freise CE, Lou CD, Garovoy MR, Bacchetti P, et al. Flow cytometry crossmatching as a predictor of acute rejection in sensitized recipients of cadaveric renal transplants. Clin Transplant. 2000; 14:167–73.
10). Gebel HM, Bray RA, Nickerson P. Pretransplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003; 3:1488–500.

11). Akalin E, Pascual M. Sensitization after kidney transplantation. Clin J Am Soc Nephrol. 2006; 1:433–40.
12). Abe M, Kawai T, Futatsuyama K, Tanabe K, Fuchinoue S, Teraoka S, et al. Postoperative production of antidonor antibody and chronic rejection in renal transplantation. Transplantation. 1997; 63:1616–9.
13). Piazza A, Borrelli L, Buonomo O, Pisani F, Valeri M, Torlone N, et al. Flow cytometry crossmatch and kidney graft outcome. Transplant Proc. 1999; 31:314–6.

14). Piazza A, Borrelli L, Monaco PI, Poggi E, Pisani F, Valeri M, et al. Posttransplant donor-specific antibody characterization and kidney graft survival. Transpl Int. 2000; 13(S1):S439–43.

15). Piazza A, Poggi E, Borrelli L, Servetti S, Monaco PI, Buonomo O, et al. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cyto-metric techniques. Transplantation. 2001; 71:1106–12.
16). Christiaans MH, Overhof-de Roos R, Nieman F, van Hooff JP, van den Berg-Loonen EM. Donor-specific antibodies after transplantation by flow cytometry: relative change in fluorescence ratio most sensitive risk factor for graft survival. Transplantation. 1998; 65:427–33.
17). Bray RA, Nickerson PW, Kerman RH, Gebel HM. Evolution of HLA antibody detection: technology emu-lating biology. Immunol Res. 2004; 29:41–54.

19). Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opi Immunol. 2005; 17:541–5.

20). Zhang Q, Liang LW, Gjertson DW, Lassman C, Wilkinson AH, Kendrick E, et al. Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation. 2005; 79:591–8.

21). Tinckam KJ, Chandraker A. Mechanisms and role of HLA and non-HLA alloantibodies. Clin J Am Soc Nephrol. 2006; 1:404–14.

22). Scornik JC, Salomon DR, Lim PB, Howard RJ, Pfaff WW. Posttransplant antidonor antibodies and graft rejection. Evaluation by two-color flow cytometry. Transplantation. 1989; 47:287–90.
23). Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004; 4:438–43.

24). Gloor JM, DeGoey S, Ploeger N, Gebel H, Bray R, Moore SB, et al. Persistence of low levels of alloantibody after desensitization in crossmatch-positive living-donor kidney transplantation. Transplantation. 2004; 78:221–27.

25). Kim SJ, et al. The 4th Japan-Korean Transplantation Forum. 2003.
26). Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, et al. Frequency and clinical implications of development of donor-specific and non-donor- specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005; 16:2804–12.
27). Oh EJ, Park YJ, Kim JY, Yang CW, Kim DG, Moon IS. Detection of Donor Specific Anti-HLA Antibodies Using Antibody Monitoring System. J Korean Soc Transplant. 2006; 20:63–8.
Go to : 
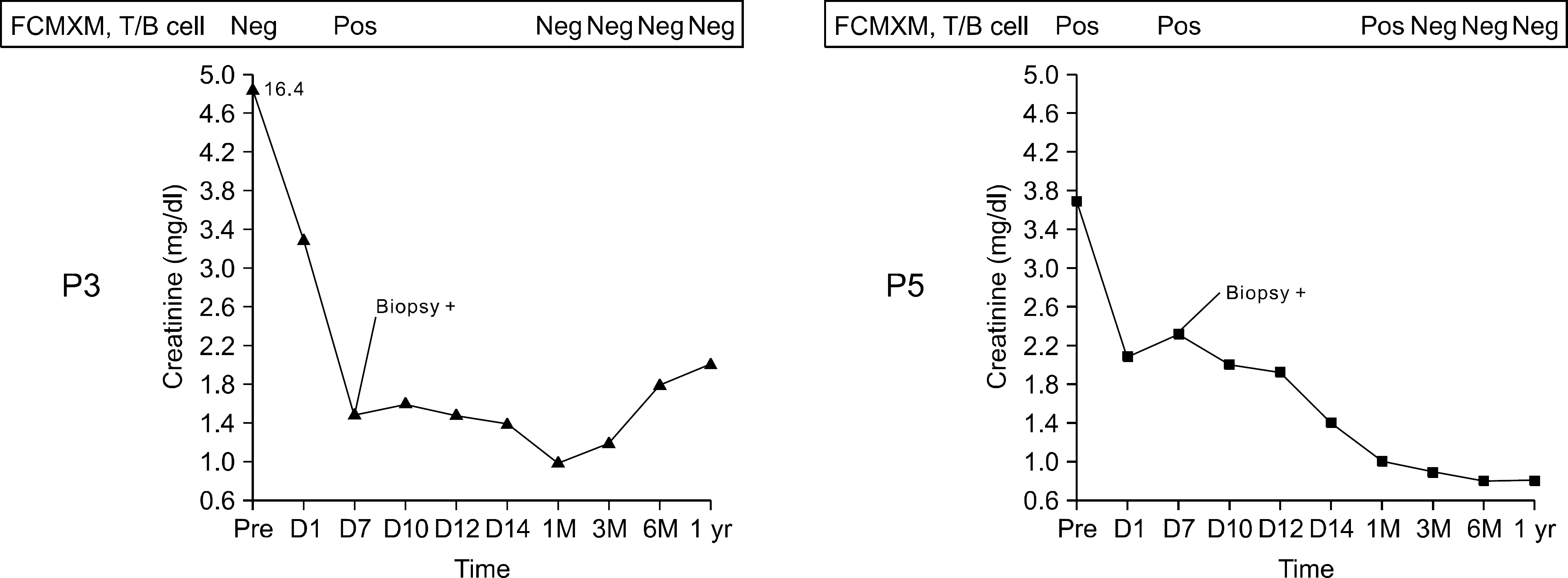 | Fig. 1.Clinical and laboratory manifestations of 2 FCXM positive patients (P3 and P5) during 1 year period post renal transplantation. |
Table 1.
Demography and clinical characteristics of patients
Table 2.
Immunologic characteristics and clinical outcomes of renal transplant patients
| Case No. |
HLA mismatches |
Pretransplant XM |
Posttransplant XM |
Specificity of Antibodies | Rejection episode | Tissue biopsy | Follow-up period (months) | Graft loss | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | DRB1 | AHG CDC | FC | FC | |||||||
| 1 | 1 | 2 | 1 | - | - | - | - | ND | 46 | - | ||
| 2 | 2 | 1 | 2 | - | - | - | - | ND | 44 | - | ||
| 3 | 2 | 1 | 1 | - | - | + | NS∗ | + | ATN, AR | 43 | - | |
| 4 | 2 | 2 | 2 | - | - | - | - | NR, drug toxicity | 39 | - | ||
| 5 | 0 | 2 | 2 | - | + | +/+† | B51‡ | + | AR | 37 | - | |
| 6 | 0 | 0 | 0 | - | - | - | - | ND | 12 | - | ||
| 7 | 1 | 1 | 1 | - | - | - | - | ND | 35 | - | ||
| 8 | 1 | 1 | 1 | - | - | - | - | Minimal tubulitis | 34 | - | ||
| 9 | 1 | 1 | 1 | - | - | - | - | ND | 26 | - | ||
| 10 | 1 | 1 | 1 | - | - | - | - | ND | 22 | - | ||
| 11 | 1 | 1 | 1 | - | - | - | - | ND | 22 | - | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download