Abstract
Diabetes mellitus is increasing all over the world and is a serious health problem. Pancreatic islet transplantation is promising treatment for diabetes mellitus, but an imbalance between deceased pancreas donors and recipients limited the widespread clinical application. Therefore, pig islets could be used as an alternative islet source in transplantation. However, a big hurdle to clinical application of islet xenotransplantation is the instant blood mediated inflammatory reaction (IBMIR), which is characterized by activation of the coagulation cascade, platelets and complement systems. Innate immune cells infiltrate the islets in the process of IBMIR and thereby accelerate the early graft loss. Characteristics of IBMIR in islet xenotransplantion are very different from the rejection in solid organ xenotransplantation. Therefore, we focus on the molecules for surmounting IBMIR in order to accomplish successful islet xenotransplantation. To prevent the IBMIR in islet xenotransplantation, development of genetic modified pigs containing anti-coagulant, anti-thrombosis and complement regulatory genes, or capsulation of islet with biomaterials for blocking immune response around islet surface can be tried. Gα-Gal knockout pigs and the diverse transgenic pigs for complement regulatory protein or anti-coagulant genes have been developed for xenotransplantation. This review summarized on characteristics of rejection in islet xenotransplantation and discusses the strategies for overcoming the rejection.
REFERENCES
1). Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001; 414:782–7.

2). Korea National Health and Nutrition Examination Survey. Korea National Health and Nutrition Examination Survey 2005 [Internet]. Seoul: The Korea Centers for Disease Control and Prevention;2009. Available from. http://knhanes.cdc.go.kr.
3). Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Falqui L, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990; 39:515–8.

4). Park JB, Kim SJ. Clinical islet transplantation: where do we stand on? J Korean Soc Transplant. 2007; 21:196–202.
5). Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343:230–8.

6). Rood PP, Buhler LH, Bottino R, Trucco M, Cooper DK. Pig-to-nonhuman primate islet xenotransplantation: a review of current problems. Cell Transplant. 2006; 15:89–104.

7). Reemtsma K, McCracken BH, Schlegel JU, Pearl MA, Pearce CW, Dewitt CW, et al. Renal heterotransplantation in man. Ann Surg. 1964; 160:384–410.

8). Bailey LL, Nehlsen-Cannarella SL, Concepcion W, Jolley WB. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA. 1985; 254:3321–9.

9). Pruitt SK, Kirk AD, Bollinger RR, Marsh HC Jr, Collins BH, Levin JL, et al. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation. 1994; 57:363–70.

10). Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005; 80:1493–500.
11). Rayat GR, Gill RG. Indefinite survival of neonatal porcine islet xenografts by simultaneous targeting of LFA-1 and CD154 or CD45RB. Diabetes. 2005; 54:443–51.

12). Choi I, Kim SD, Cho B, Kim D, Park D, Koh HS, et al. Xenogeneic interaction between human CD40L and porcine CD40 activates porcine endothelial cells through NF-kappaB signaling. Mol Immunol. 2008; 45:575–80.
13). Ahn C, et al. Fgl2 induction in porcine endothelial cells through xenogeneic CD40-CD40L interaction. (in press).
14). Lee EM, Park JO, Kim D, Kim JY, Oh KH, Park CG, et al. Early up-regulation of CXC-chemokine expression is associated with strong cellular immune responses to murine skin xenografts. Xenotransplantation. 2006; 13:328–36.

15). Kim JY, Kim D, Lee EM, Choi I, Park CG, Kim KS, et al. Inducible nitric oxide synthase inhibitors prolonged the survival of skin xenografts through selective down- regulation of pro-inflammatory cytokine and CC-chemo-kine expressions. Transpl Immunol. 2003; 12:63–72.
16). Yang J, Cho B, Choi I, Kim DH, Kim SD, Koh HS, et al. Molecular characterization of miniature porcine RANTES and its chemotactic effect on human mononuclear cells. Transplantation. 2006; 82:1229–33.

17). Yang J, Choi I, Kim SD, Kim ES, Cho B, Kim JY, et al. Molecular characterization of cDNA encoding porcine IP-10 and induction of porcine endothelial IP-10 in response to human TNF-alpha. Vet Immunol Immunopathol. 2007; 117:124–8.
18). Choi I, Cho B, Kim SD, Park D, Kim JY, Park CG, et al. Molecular cloning, expression and functional characterization of miniature swine CD86. Mol Immunol. 2006; 43:480–6.

19). Nilsson B. The instant blood-mediated inflammatory reaction in xenogeneic islet transplantation. Xenotransplantation. 2008; 15:96–8.

20). Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000; 105:125–33.

21). Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999; 48:1907–14.

22). Bühler L, Deng S, O'Neil J, Kitamura H, Koulmanda M, Baldi A, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002; 9:3–13.

23). van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007; 14:288–97.

25). Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008; 86:702–9.

26). Glaser CB, Morser J, Clarke JH, Blasko E, McLean K, Kuhn I, et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modu-lation of coagulation. J Clin Invest. 1992; 90:2565–73.

27). Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-throm-bomodulin complex. Proc Natl Acad Sci USA. 1996; 93.

28). Taylor FB Jr, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an im-portant role in protein C activation in vivo. Blood. 2001; 97:1685–8.
29). Pike RN, Buckle AM, Le Bonniec BF, Church FC. Control of the coagulation system by serpins. Getting by with a little help from glycosaminoglycans. FEBS J. 2005; 272:4842–51.
30). Dwyer KM, Mysore TB, Crikis S, Robson SC, Nandurkar H, Cowan PJ, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006; 82:428–32.

31). Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007; 3:171–80.

32). Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galacto-syltransferase gene-knockout pigs in baboons. Am J Pathol. 2008; 172:1471–81.
33). Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human an-ti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993; 1:198–205.
34). Rood PP, Bottino R, Balamurugan AN, Smetanka C, Ayares D, Groth CG, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007; 83:202–10.

35). Rayat GR, Rajotte RV, Hering BJ, Binette TM, Korbutt GS. In vitro and in vivo expression of Galalpha-(1,3)Gal on porcine islet cells is age dependent. J Endocrinol. 2003; 177:127–35.
36). Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, et al. Transplantation of cul-tured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004; 4:390–401.

37). Mohanakumar T, Narayanan K, Desai N, Ramachandran S, Shenoy S, Jendrisak M, et al. A significant role for histocompatibility in human islet transplantation. Transplantation. 2006; 82:180–7.

38). Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006; 12:682–7.

39). Janeway CA Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. 6th ed.New York: Garland Science;2005. p. 55–75.
40). Kues WA, Schwinzer R, Wirth D, Verhoeyen E, Lemme E, Herrmann D, et al. Epigenetic silencing and tissue in-dependent expression of a novel tetracycline inducible system in double-transgenic pigs. FASEB J. 2006; 20:1200–2.

41). Cowan PJ, Aminian A, Barlow H, Brown AA, Chen CG, Fisicaro N, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000; 27:69. 2504–15.

42). Bennet W, Björkland A, Sundberg B, Brandhorst D, Brendel MD, Richards A, et al. Expression of complement regulatory proteins on islets of Langerhans: a com-parison between human islets and islets isolated from normal and hDAF transgenic pigs. Transplantation. 2001; 72:312–9.
43). van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normogly-cemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009; 9:2716–26.

44). Shrivastava S, McVey JH, Dorling A. The interface between coagulation and immunity. Am J Transplant. 2007; 7:499–506.

45). Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, et al. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes. 2004; 53:2804–14.
47). Moberg L, Korsgren O, Nilsson B. Neutrophilic gran-ulocytes are the predominant cell type infiltrating pancreatic islets in contact with ABO-compatible blood. Clin Exp Immunol. 2005; 142:125–31.

48). Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002; 51:1779–84.
49). Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004; 53:2559–68.

50). Hanley S, Liu S, Lipsett M, Castellarin M, Rosenberg L, Tchervenkov J, et al. Tumor necrosis factor alpha production by human islets leads to postisolation cell death. Transplantation. 2006; 82:813–18.
51). Langford GA, Yannoutsos N, Cozzi E, Lancaster R, Elsome K, Chen P, et al. Production of pigs transgenic for human decay accelerating factor. Transplant Proc. 1994; 26:1400–1.
52). Murakami H, Nagashima H, Takahagi Y, Miyagawa S, Fujimura T, Toyomura K, et al. Transgenic pigs expressing human decay-accelerating factor regulated by porcine MCP gene promoter. Mol Reprod Dev. 2002; 61:302–11.

53). Zhou CY, McInnes E, Copeman L, Langford G, Parsons N, Lancaster R, et al. Transgenic pigs expressing human CD59, in combination with human membrane cofactor protein and human decay-accelerating factor. Xenotransplantation. 2005; 12:142–8.

54). Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, et al. Production of alpha-1,3-gal-actosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002; 295:1089–92.
55). Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyl-transferase-deficient pigs. Science. 2003; 299:411–4.
56). Tu CF, Hsieh SL, Lee JM, Yang LL, Sato T, Lee KH, et al. Successful generation of transgenic pigs for human decay-accelerating factor and human leucocyte antigen DQ. Transplant Proc. 2000; 32:913–5.

57). Ramsoondar JJ, Macháty Z, Costa C, Williams BL, Fodor WL, Bondioli KR. Production of alpha 1,3-galactosyl-transferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod. 2003; 69:437–45.
58). Lee S, Chung J, Ha IS, Yi K, Lee JE, Kang HG, et al. Hydrogen peroxide increases human leukocyte adhesion to porcine aortic endothelial cells via NFkappaB-depend-ent up-regulation of VCAM-1. Int Immunol. 2007; 19:1349–59.
59). Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of antioxidant and anti-apoptotic genes. Nat Med. 1998; 4:1392–6.

60). de Vos P, Faas MM, Strand B, Calafiore R. Alginate- based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006; 27:5603–17.
61). Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL. Surface re-engineering of pancreatic islets with re-combinant azido-thrombomodulin. Bioconjug Chem. 2007; 18:1713–5.

62). Kin T, O'Neil JJ, Pawlick R, Korbutt GS, Shapiro AM, Lakey JR. The use of an approved biodegradable polymer scaffold as a solid support system for improvement of islet engraftment. Artif Organs. 2008; 32:990–3.

63). Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P Jr, et al. Long-term survival of non-human primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009; 9:91–104.

64). Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005; 11:1323–31.

65). Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006; 12:301–3.

Fig. 2.
Three stages of graft rejection in xenotransplantation. (A) Hyperacute rejection: Hyperacute rejection of pig organs in humans is induced by binding of preformed human 'natural' antibody, which is generally directed against Gala1-3Gal that exists on the surface of pig cells. Complement is activated by antibody binding, and triggers lysis of endothelial cells. (B) Acute vascular rejection: Acute vascular rejection is induced by interactions between the graft endothelial cells and host antibodies, macrophages, and platelets. The response is characterized by an inflammatory infiltrate of mostly macrophages and natural killer cells (with small numbers of T cells), intravascular thrombosis, and fibrinoid necrosis of vessel walls. (C) Acute cellular rejection: Cellular rejection is caused by cellular immunity i) Natural killer cells, which accumulate in and damage the xenograft, and ii) T-lymphocytes, which are activated by MHC molecules through both direct and indirect xenorecognition. Abbreviations: MHC, major histocompatibility complex.
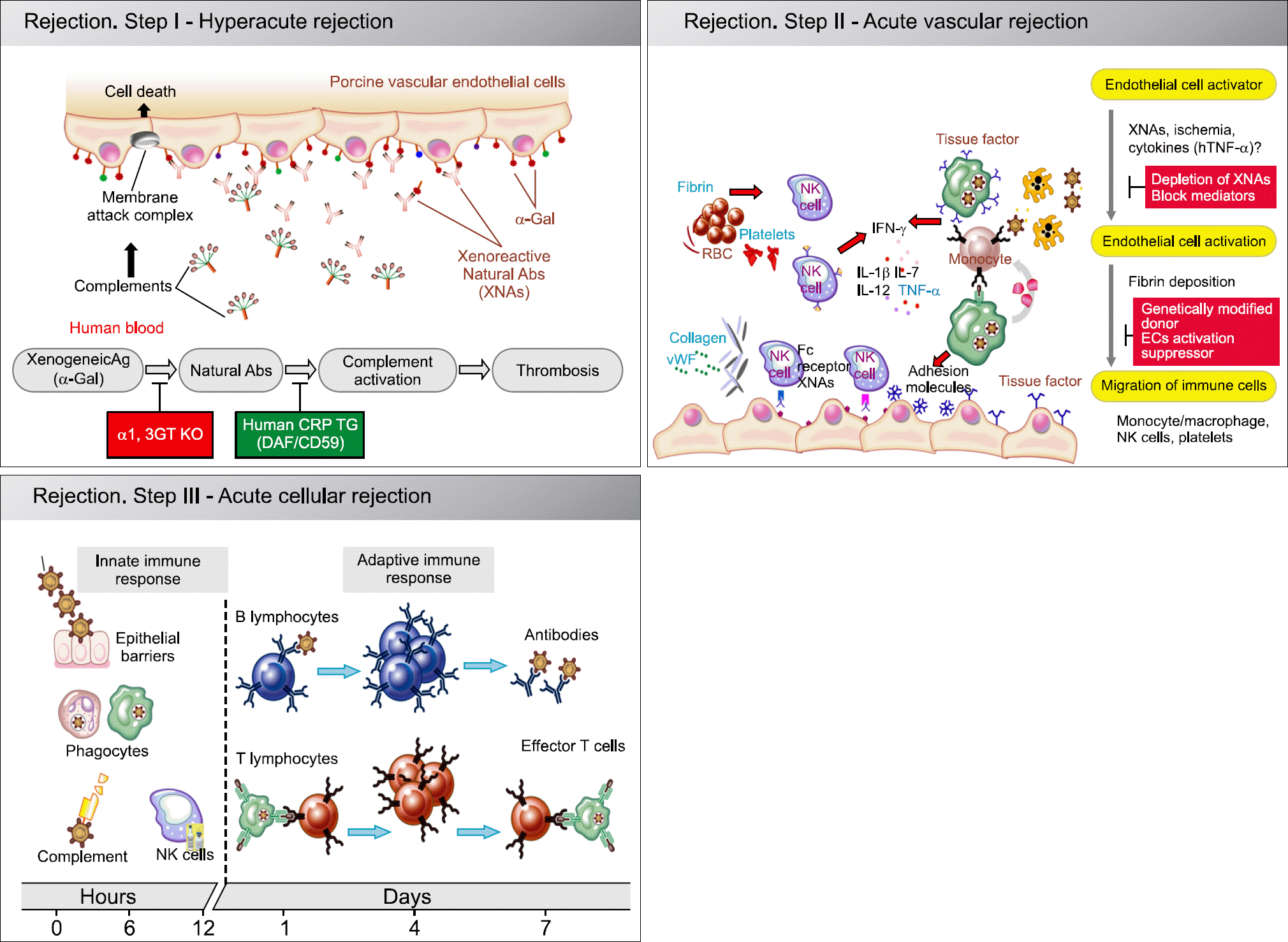
Fig. 3.
Putative model for the IBMIR. (A) In contact with blood, IgG and IgM antibodies bind to the islet surface and activate complement which leads to deposition of C3b/iC3b to the surface. (B) Tissue factor (TF) activates the coagulation system via the extrinsic pathway. As a consequence of extrinsic pathway activation, prothrombin is cleaved into thrombin. Thrombin subsequently generates fibrin and activates platelets. (C) Platelet activation increases the affinity of the integrins GPIIb-IIIa and a2b1 for fibrin and collagen, respectively. Activated platelets bind to fibrin and collagen on the islet surface. (D) Amplified by platelets, thrombin generates more fibrin creating a capsule containing platelets, PMNs, and monocytes surrounding the islets. Chemotactic factors (e.g., C5a and IL-8) that were released as a consequence of IBMIR or released directly from the islets (e.g., MCP-1, IL-8 etc.), exert their action on PMNs and monocytes that infiltrate the islets in large numbers after 30 min. Abbreviations: IBMIR, instant blood mediated inflammatory reaction; IL-8, Interleukin-8; PMNs, polymorphonuclear leukocytes; MCP-1, monocyte chemoattractant protein-1. Source: Nilson B. The instant blood-mediated inflammatory reaction in xenogeneic islet transplantation. Xenotransplantation 2008;15:96-8. p.97.
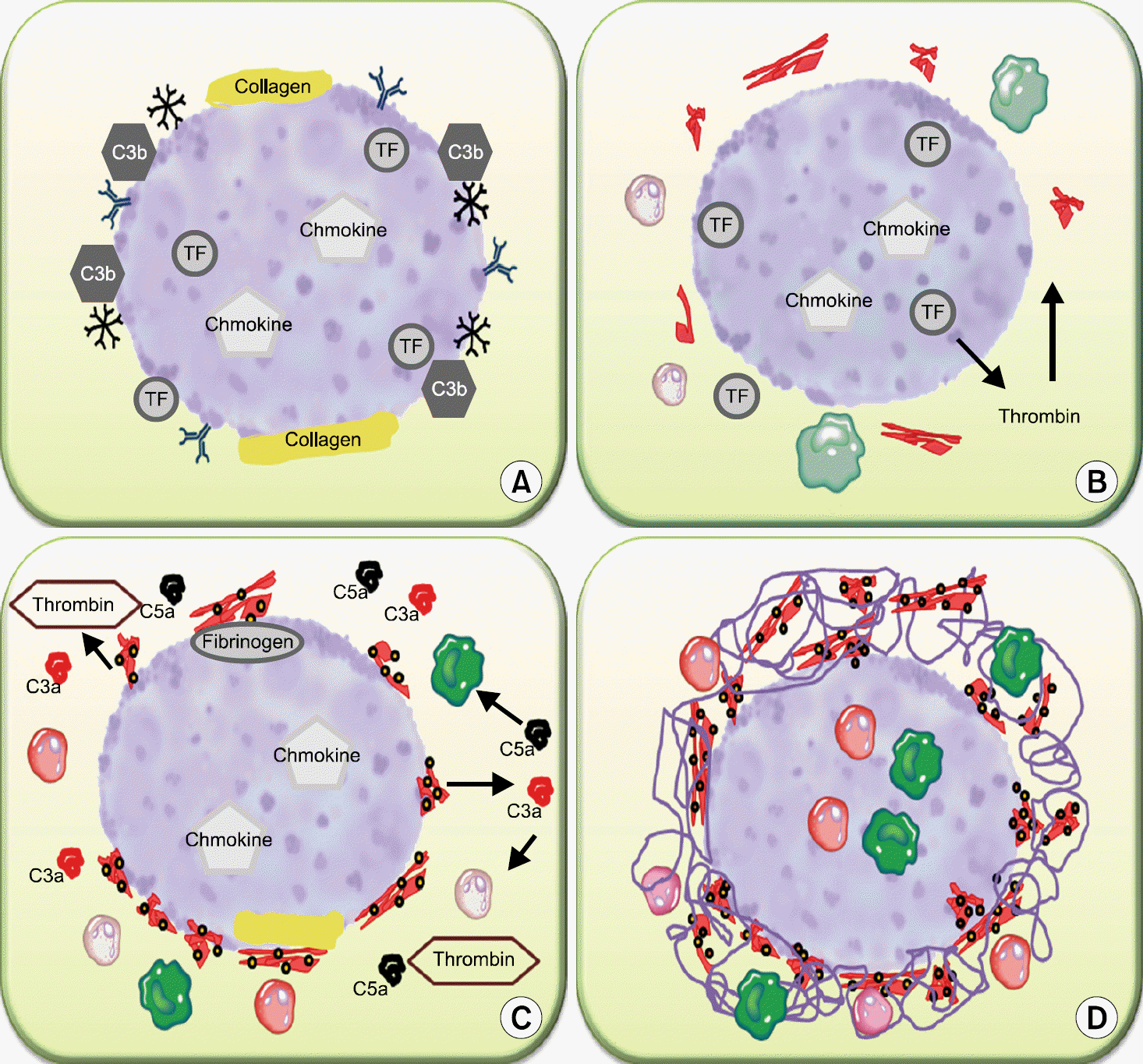
Fig. 4.
Schematic representation of the coagulation pathways. 'a' present the activated clotting factors. Briefly, coagulation is initiated when islet-expressed tissue factor (TF) is exposed to the blood in IBMIR. TF then complexes with VIIa and enhances its activity. This sequence of coagulation activation is known as the extrinsic pathway. The complex of VIIa/TF activates factors IXa and Xa, which mediate the conversion of prothrombin into the active thrombin. Nevertheless, the small quantity of thrombin formed is sufficient to activate XIa, which reinforces thrombin generation by activating the intrinsic pathway. Furthermore, the intrinsic pathway can be activated by collagen residues or other negatively charged molecules on the islet surface. Thrombin, a potent platelet activator, cleaves fibrinogen into fibrin monomers, and activates the coagulation factor that cross-links fibrin monomers into an insoluble throm-bus (XIIIa, not shown). Coagulation systems can be modulated by anti-coagulant molecules as TBM (thrombomodulin), TFPI (tissue factor pathway inhibitor), AT (antithrombin) and CD39 (ectoATPase). Source: van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 2007;14:288–97. p.289.
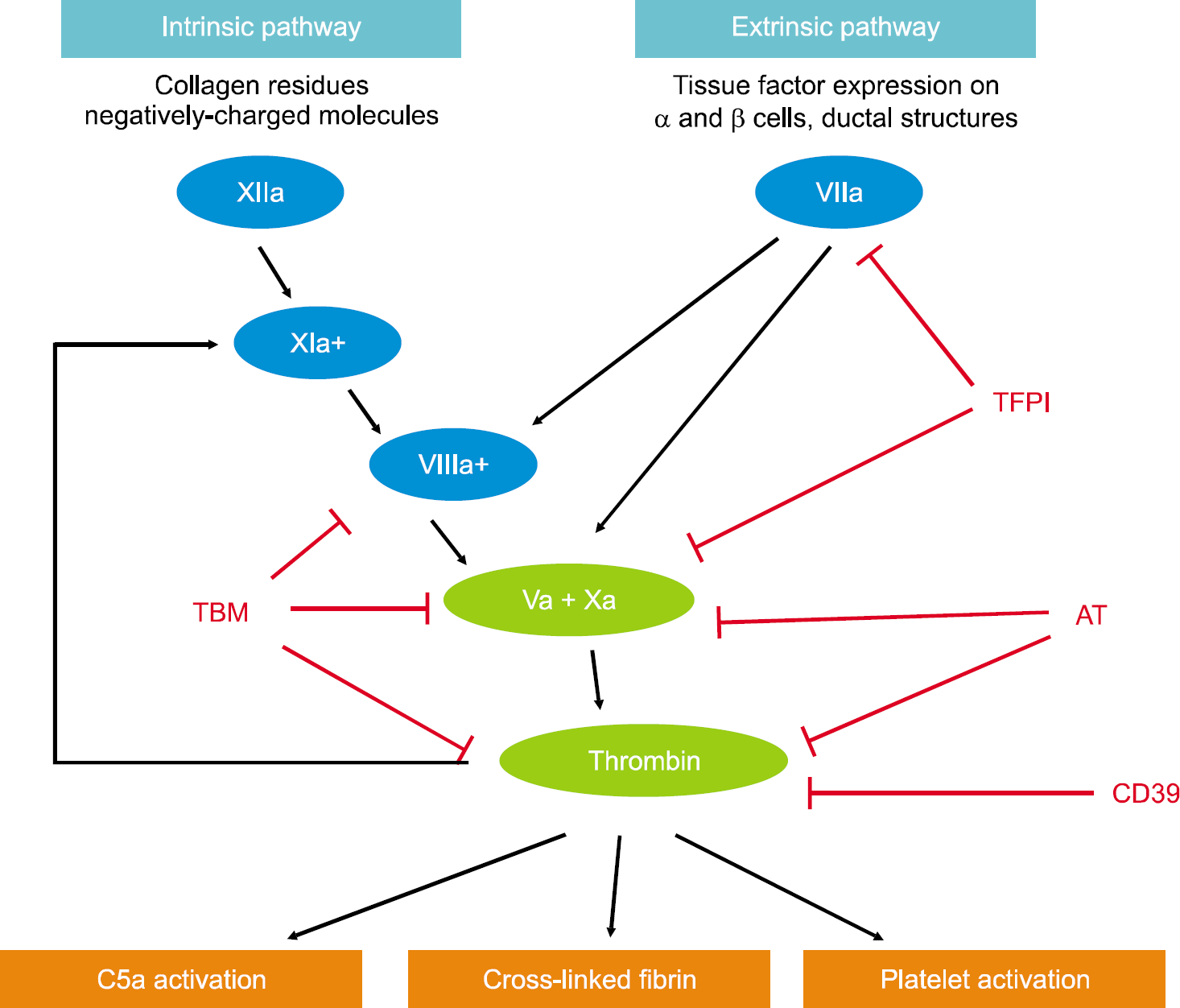
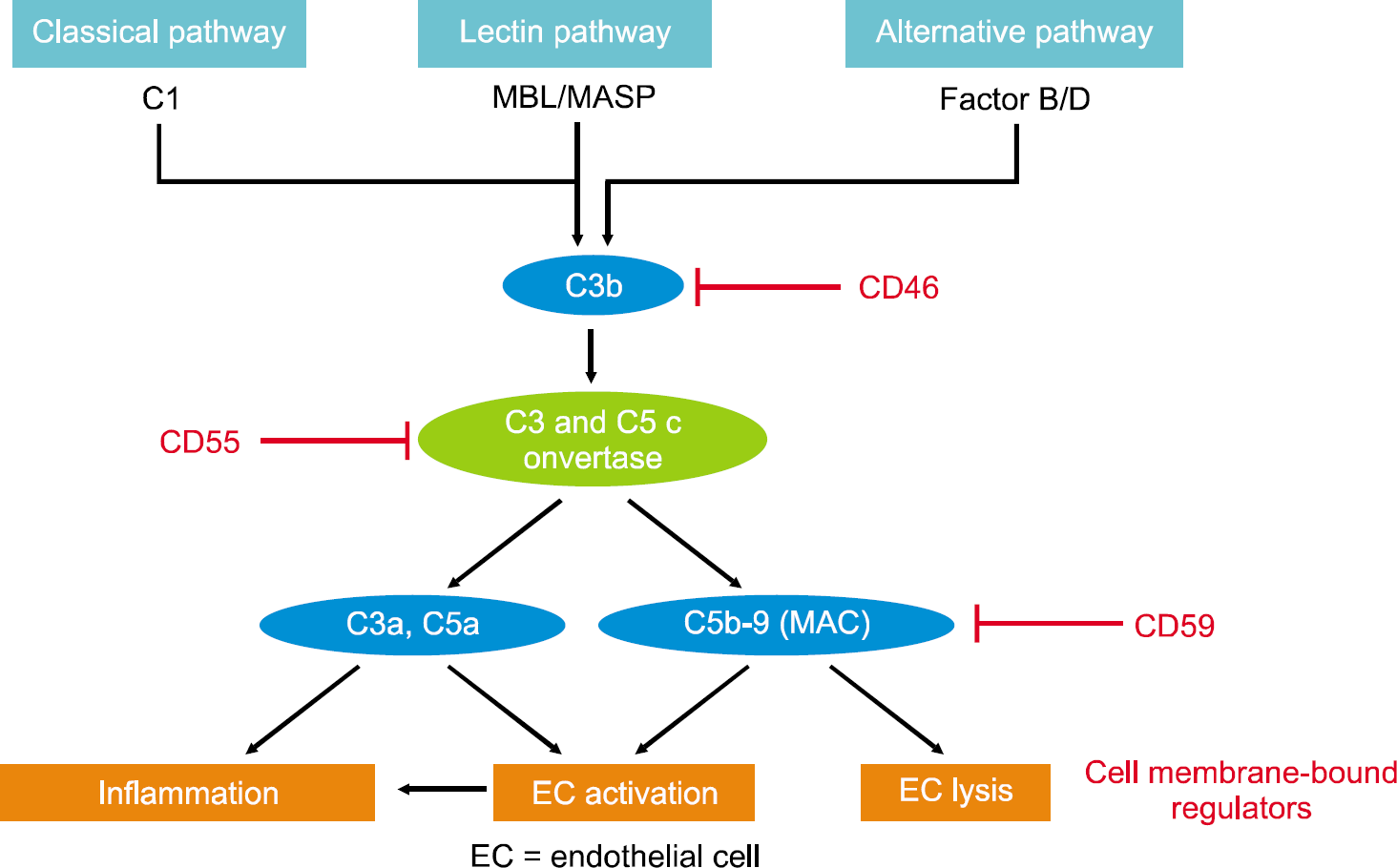
Fig. 5.
Schematic representation of the complement cascades. The membrane attack complex is initiated when C5 convertase cleaves C5 into C5a and C5b. After C6, binds to C5b, the C5bC6 complex is bound by C7. This junction alters the configuration of the protein molecules exposing a hydrophobic site on C7 that allows the C7 to insert into the phospholipid bilayer of the pathogen. Similar hydrophobic sites on C8 and C9 molecules are exposed when they bind to the complex, so that they can also insert into the bilayer. The ring structure formed by C9 is a pore in the membrane that allows free diffusion of molecules in and out of the cell. If enough pores form, the cell is no longer able to survive. There is a membrane bound complement regulator such as DAF (CD55), CD59, and MCP (CD46). CD46 is an inhibitory complement receptor and decay accelerating factor (CD55) is a 70 kDa membrane protein that regulates the complement system on the cell surface. CD59 inhibits the complement membrane attack complex by binding C5b678 and preventing C9 from binding and polymerizing. Abbreviations: MBL, Mannose binding lectin; MASP, MBL-associated serine protease; DAF, decay accelerating factor; MCP, membrane cofactor pretein. Source: van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 2007;14:288–97. p.290.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download