Abstract
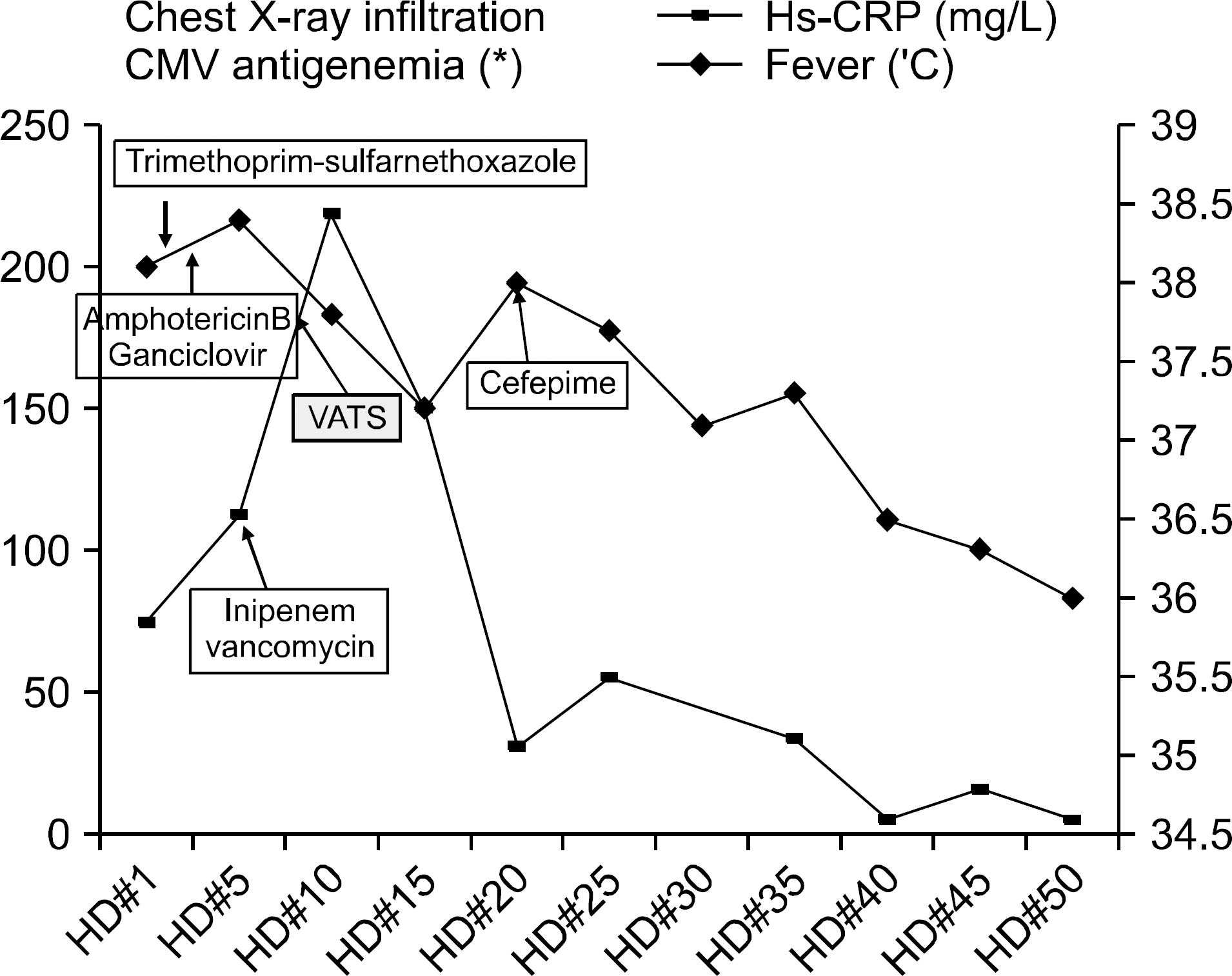
Renal transplantation has become a well-established, definitive, highly successful therapy for end stage renal disease and been increased in previous decades. Korean Network for Organ Sharing reports that renal transplantation has been performed over 800 cases per year during five years. Although graft survival after renal transplantation has increased with the develop-ment of numerous new immunosupressive agents, infectious complications remain a significant cause of morbidity and mortality in renal transplant recipients.Cytomegalovirus (CMV) is a major virus in organ transplant recipients and is associated with opportunistic superinfection with a range of different microorganisms including Pneumocystis jirovecii, fungi, gram neg-ative bacterias. In this paper, we report a case of pneumonia caused by fungus, Pneumocystis jirovecii, CMV in patient with renal transplantation. Based on the strong suspicion of superinfection, we aggressively diagnosed by performing surgical method and successfully treated the condition. Patients with CMV pneumonitis may be predisposed to superinfection by other pathogen and is associated with high mortality. Therefore, if superinfection is suspected, prompt diagnosis involving invasive methods and early initiation of antiviral, antifungal therapy are essential to reduce the mortality.
REFERENCES
1). Rao KV, Anderson RC. Long-term results and complications in renal transplant recipients; observations in the second decade. Transplantation. 1988; 45:45–52.

2). Flechner SM, Payne WD, Van Buren C, Kerman R, Kahan BD. The effect of cyclosporine on early graft function in human renal transplantation. Transplantation. 1983; 36:268–72.

3). Koo TY, Park HS, Kim HC, Park JS, Lee CH, Kim GH, et al. Infectious complications in patients with kidney transplantation: follow-up results in single center. J Korean Soc Transplant. 2008; 22:77–84.
4). Hwang EA, Lee KT, Park KD, Park SB, Kim HC, Jo WH, et al. Infections following renal transplantation: long term follow-up study in single center. Korean J Nephrol. 2000; 19:713–23.
5). Ketteler M, Preuschof L, Mertz A, Stöffler-Meilicke M, Schäfer H, Distler A, et al. Fatal cytomegalovirus pneumonia after preemptive antiviral therapy in a renal transplant recipient. Clin Nephrol. 2000; 54:418–24.
6). Rubin RH, Wolfson JS, Cosimi AB, Tolkoff-Rubin NE. Infection in the renal transplant recipient. Am J Med. 1981; 70:405–11.

7). Masur H, Cheigh JS, Stubenbord WT. Infection following renal transplantation: A changing pattern. Rev Infect Dis. 1982; 4:1208–19.

8). Bittner K, Bittinger A, Lange H. Cytomegalovirus-asso-ciated superinfection of the lung following kidney transplantation. Dtsch Med Wochenschr. 1987; 112:214–8.
9). Chugh KS, Sakhuja V, Jain S, Talwar P, Minz M, Joshi K, et al. High mortality in systemic fungal infections following renal tranaplantation in third world countries. Nephrol Dial Transplant. 1993; 8:168–72.
10). Sia IG, Paya CV. Infectious complications following renal transplantation. Surg Clin North Am. 1998; 78:95–112.

11). Song JH. Infectious complications in solid organ transplantation. Infection. 1996; 28:1–14.
12). Stevens DA, Kan VL, Judson MA, Morrison VA, Du-mmer S, Denning DW, et al. Practice guidelines for disease caused by Aspergillus. Infectious Diseases Society of America. Clin Infect Dis. 2000; 30:696–709.
13). Kim YJ, Kim SI, Kim YR, Yang CW, Kang MW, Bang BK. Two successfully treated cases of posttransplant pneumonia caused by cytomegalovirus and aspergillus coinfection. J Korean Soc Transplant. 2008; 22:130–4.
Fig. 1.
(A) Chest radiograph shows peribronchial infiltration in both lung fields especially left. (B) Chest radiograph obtained 13 days later shows decreased bilateral infiltration.
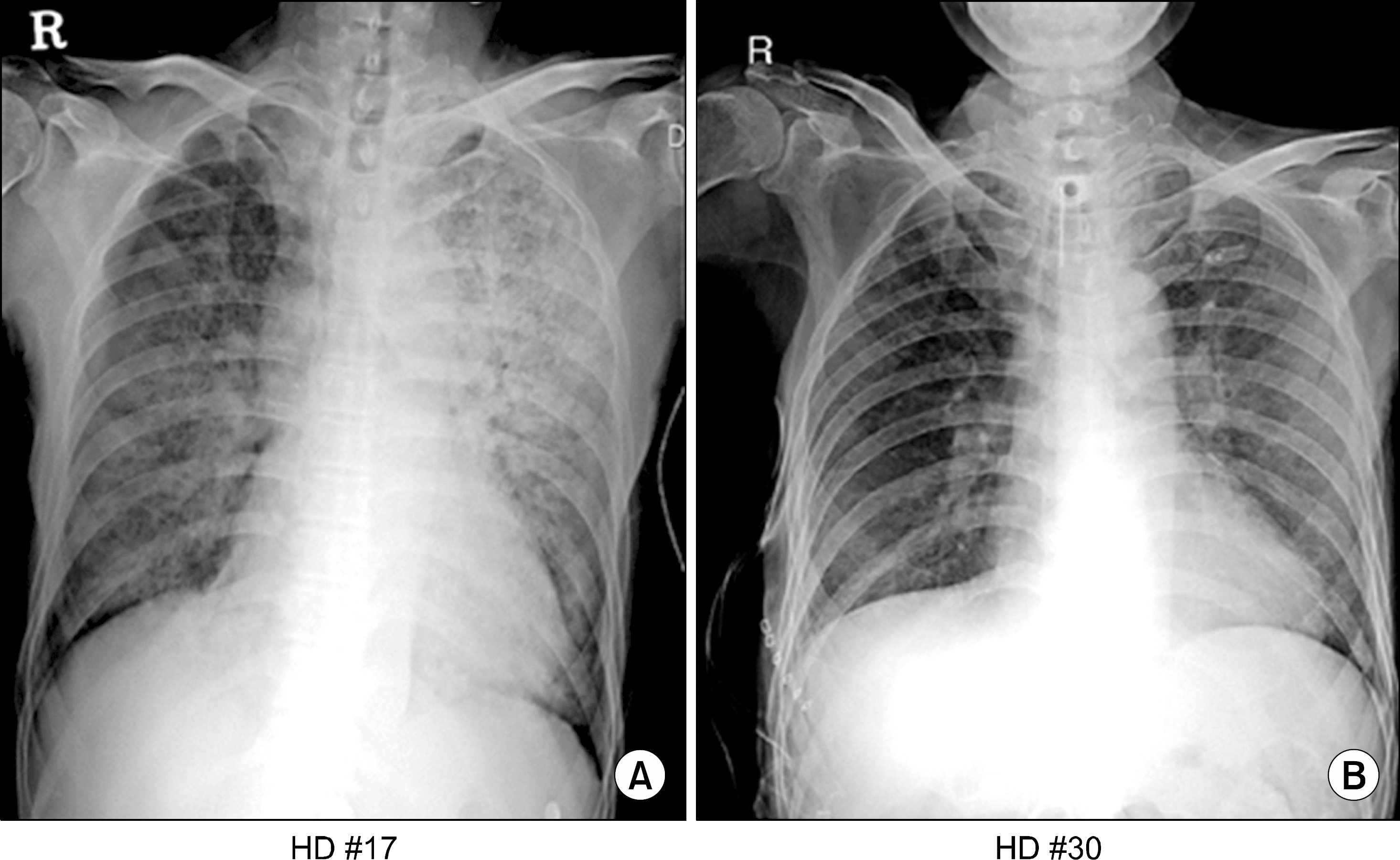
Fig. 2.
(A) HRCT section through the lung bases shows multiple consoliations and diffuse ground glass opacification in both lower lungs. (B) HRCT obtained 13 days later shows interval improvement with diffuse gound glass opacification and cosolidations in both lungs.
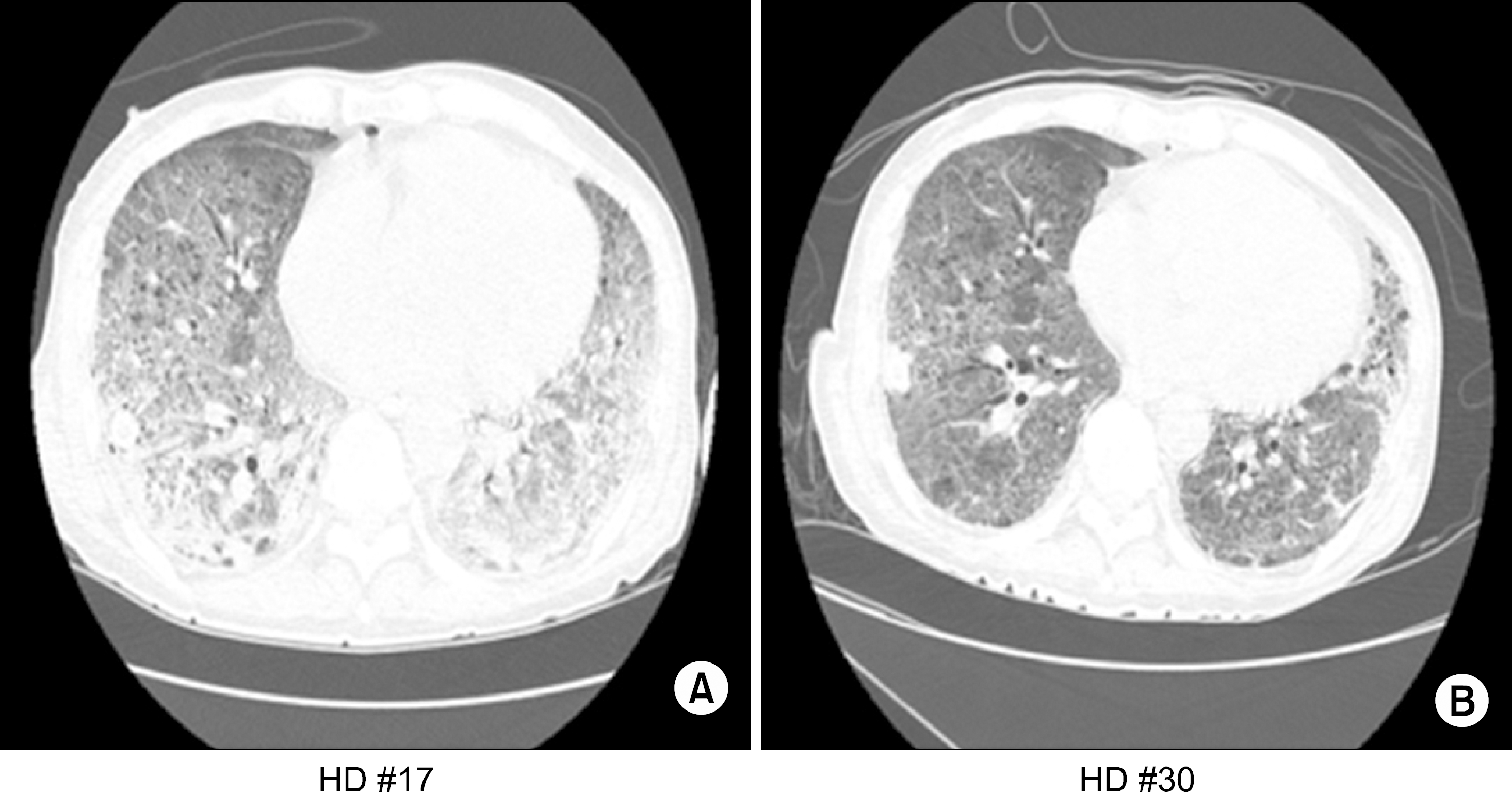
Fig. 3.
(A) Hematoxylin eosin staining (×400) showing the infla-mmatory cell infiltration in the in-terstitium and exudation in alveo-lar space. (B) Immunohistochemi-cal staining for CMV (×400) showing positive, round cells (C) Gomori methenamine silver staining (×400) showing the wall of pneumocystis cyst. (D) Periodic acid-schiff stain-ing (PAS, ×400) showing myecelia, spore that are suggestive of fungi.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download