Abstract
Biofilms are commonly associated with an increased risk of catheter-associated infection. To study the efficacy of materials designed to reduce biofilm formation, microbial biofilms on clinically used urinary catheter were examined. We performed 2, 3-bis (2-methyoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay to determine of biofilm formation ability and observed with scanning electron microscopy (SEM) to analyze biofilm architecture. Additionally, we calculated relative cell surface hydrophobicity (CSH) to measure hydrophobicity of microorganisms. On SEM, catheter surfaces made of latex or anti-infective (IC)-latex were rough but those of silicone, hydrogel-coated silicone (HCS), or silver-alloy-coated silicone (SCS) were relatively smoother. According to XTT reduction assay, biofilm formation was reduced on the surface of smooth silicone-based catheters compared to rough latex-based catheters. The greatest to lowest formation of microbial biofilm were as follows for these material types: silicone-elastomer-coated (SEC) latex > latex > silicone > IC-latex > HCS > SCS. Catheter materials can affect the microbial biofilm formations. First, rougher surfaces on the catheter made the microbial attachment easier and a greater amount of biofilm was formed. Second, when chemicals that inhibit growth and attachment of microorganisms on the inner and outer surfaces of the catheters were applied, the biofilm formation was inhibited. SCS was found to be the most effective in reducing the microbial biofilm formation. These results indicate that microbial biofilm formation may be closely related to the surface roughness and microbial CSH.
Go to : 
REFERENCES
1). Trautner BW, Darouiche RO. Catheter-associated infections: pathogenesis affects prevention. Arch Intern Med. 2004; 164:842–50.
2). Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982; 307:637–42.
3). Lo E, Nicolle L, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008; 29(Suppl 1):S41–50.

4). Srinivasan A, Karchmer T, Richards A, Song X, Perl TM. A prospective trial of a novel, silicone-based, silver-coated foley catheter for the prevention of nosocomial urinary tract infections. Infect Control Hosp Epidemiol. 2006; 27:38–43.

5). Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002; 15:167–93.

7). Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999; 74:131–6.

8). Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006; 9:340–5.
10). Belfield PW. Urinary catheters. Br Med J (Clin Res Ed). 1988; 296:836–7.
11). Lynch AS, Robertson GT. Bacterial and fungal biofilm infections. Annu Rev Med. 2008; 59:415–28.

12). Schumm K, Lam TB. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database Syst Rev. 2008. CD004013.

13). Lawrence EL, Turner IG. Materials for urinary catheters: a review of their history and development in the UK. Med Eng Phys. 2005; 27:443–53.

14). Ramage G, Vandewalle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001; 18:163–70.
15). Rosenberg M. Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol Lett. 1984; 22:289–95.

16). Klotz SA, Drutz DJ, Zajic JE. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985; 50:97–101.
17). Hazen KC, Brawner DL, Riesselman MH, Jutila MA, Cutler JE. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991; 59:907–12.
18). Patel JD, Ebert M, Stokes K, Ward R, Anderson JM. Inhibition of bacterial and leukocyte adhesion under shear stress conditions by material surface chemistry. J Biomater Sci Polym Ed. 2003; 14:279–95.

19). Ahearn DG, Grace DT, Jennings MJ, Borazjani RN, Boles KJ, Rose LJ, et al. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infections. Curr Microbiol. 2000; 41:120–5.
20). Gabriel MM, Mayo MS, May LL, Simmons RB, Ahearn DG. In vitro evaluation of the efficacy of a silver-coated catheter. Curr Microbiol. 1996; 33:1–5.
21). Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014; 4:112.

22). Silva-Dias A, Miranda IM, Branco J, Monteiro-Soares M, Pina-Vaz C, Rodrigues AG. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal plank tonic susceptibility: relationship among Candida spp. Front Microbiol. 2015; 6:205.

23). Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol. 2003; 69:4278–81.

24). Wang R, Neoh KG, Shi Z, Kang ET, Tambyah PA, Chiong E. Inhibition of Escherichia coli and Proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol Bioeng. 2012; 109:336–45.
25). Beattie M, Taylor J. Silver alloy vs. uncoated urinary catheters: a systematic review of the literature. J Clin Nurs. 2011; 20:2098–108.

26). Desai DG, Liao KS, Cevallos ME, Trautner BW. Silver or nitrofurazone impregnation of urinary catheters has a minimal effect on uropathogen adherence. J Urol. 2010; 184:2565–71.

27). Davenport K, Keeley FX. Evidence for the use of silver-alloy-coated urethral catheters. J Hosp Infect. 2005; 60:298–303.
Go to : 
 | Figure 1.Scanning electron micrographs of the catheter surface. (A) Latex, (B) anti-infective latex (IC-Latex), (C) silicone-elastomer-coated latex (SEC), (D) 100% silicone, (E) hydrogel-coated silicone (HCS), (F) silver-alloy-coated silicone (SCS). (scale bar, 10 μm) |
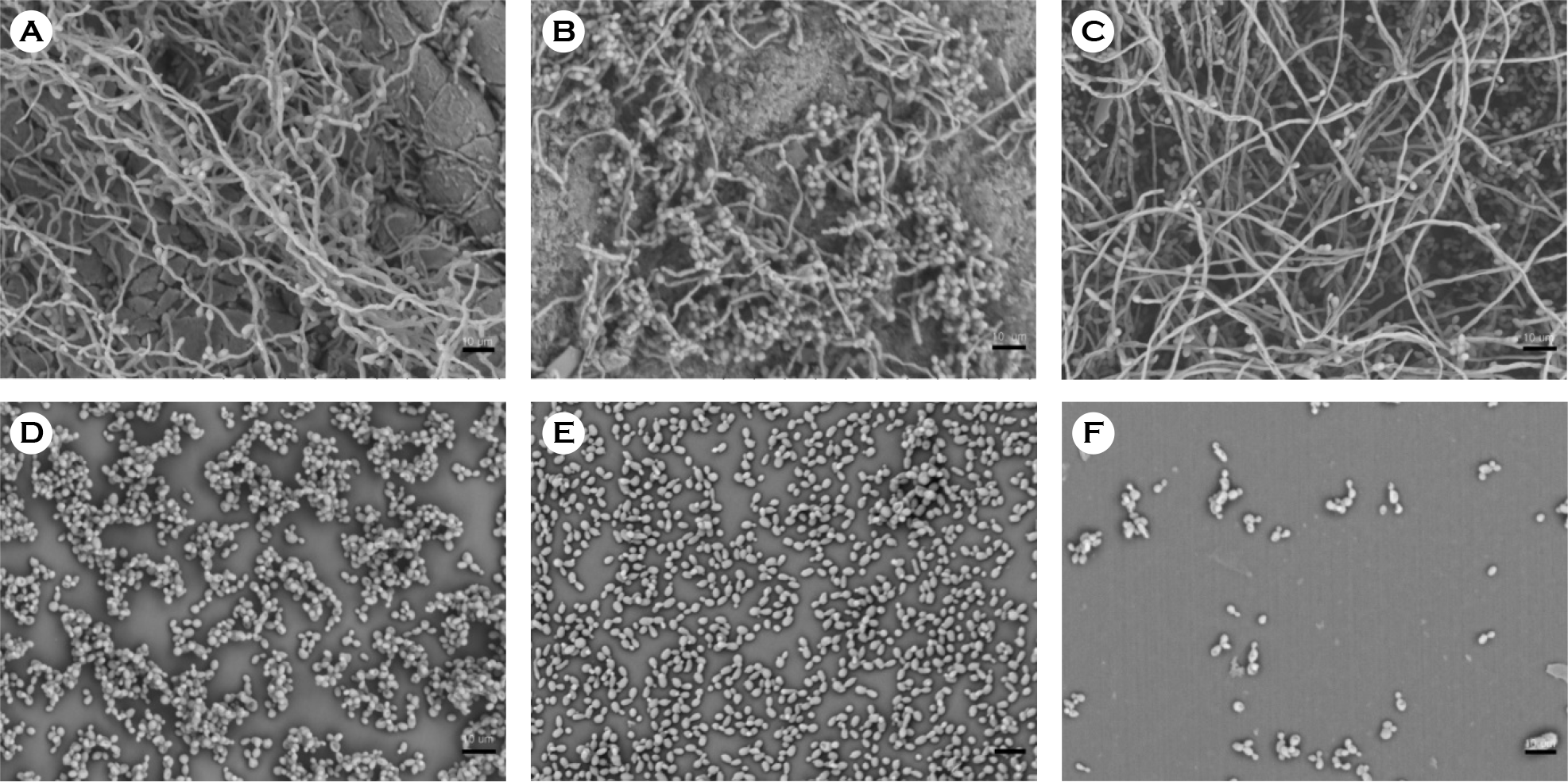 | Figure 2.Scanning electron micrographs of biofilm formation by C. albicans on catheter discs. (A) Latex, (B) anti-infective latex (IC-Latex), (C) silicone-elastomer coated latex (SEC), (D) 100% silicone, (E) hydrogel-coated silicone (HCS), (F) silver-alloy-coated silicone (SCS). (scale bar, 10 μm) |
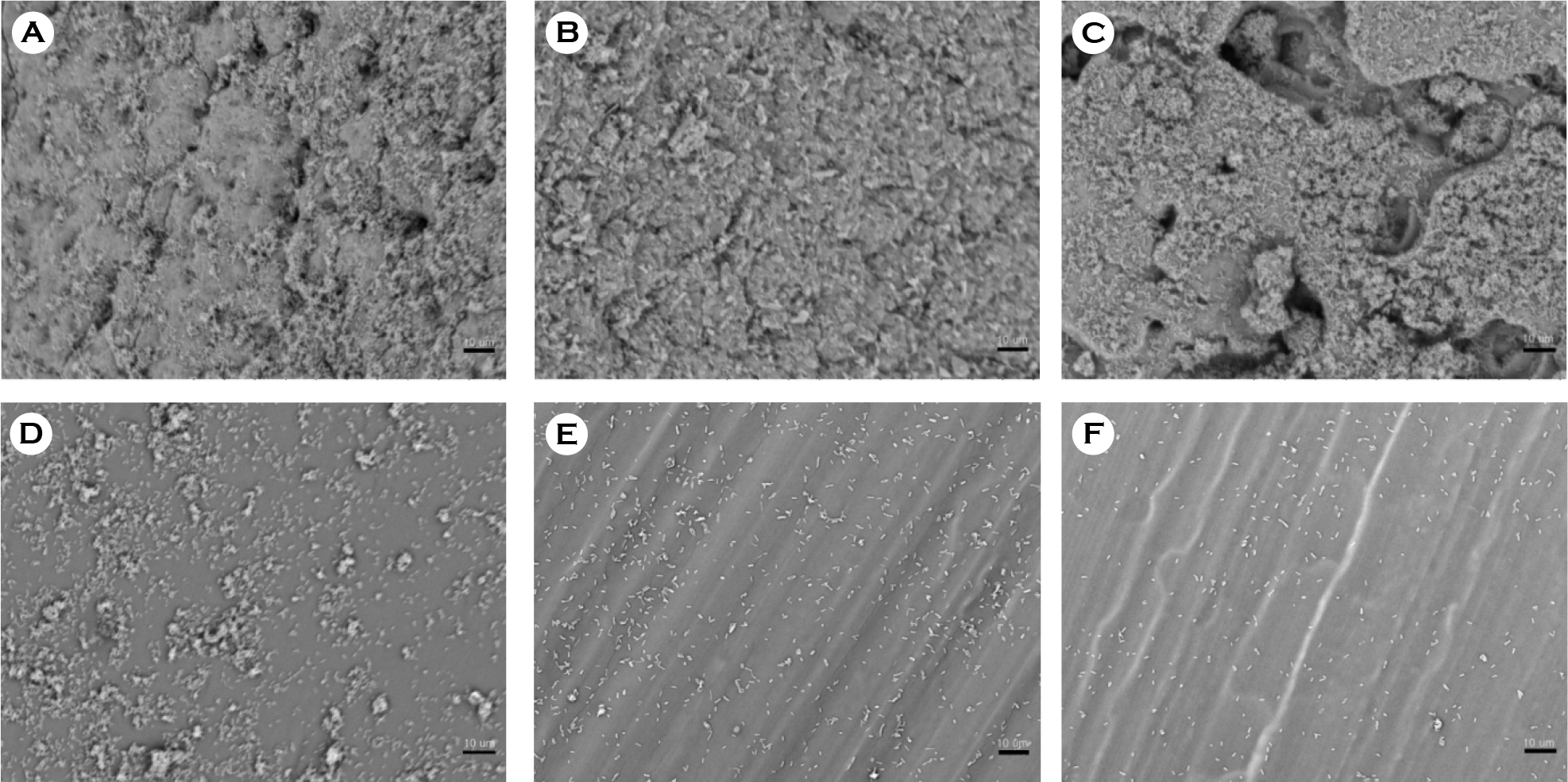 | Figure 3.Scanning electron micrographs of biofilm formation by E. coli on catheter discs. (A) Latex, (B) anti-infective latex (IC-Latex),(C) silicone-elastomer coated latex (SEC), (D) 100% silicone, (E) hydrogel-coated silicone (HCS), (F) silver-alloy-coated (SCS). (scale bar, 10 μm) |
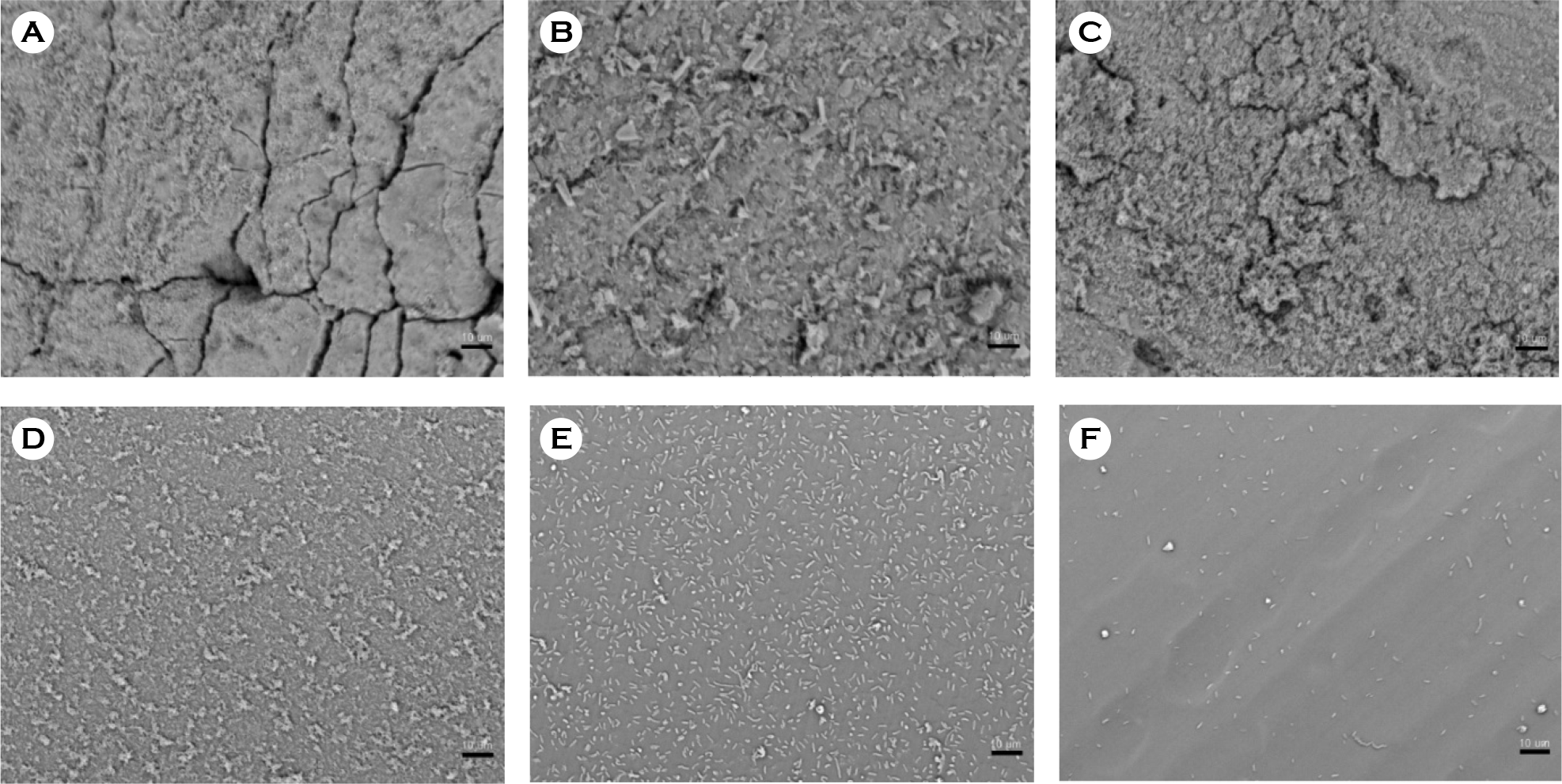 | Figure 4.Scanning electron micrographs of biofilm formation by P. vulgaris on catheter discs. (A) Latex, (B) anti-infective latex (IC-Latex), (C) silicone-elastomer coated latex (SEC), (D) 100% silicone, (E) hydrogel-coated silicone (HCS), (F) silver-alloy-coated silicone (SCS). (scale bar, 10 μm) |
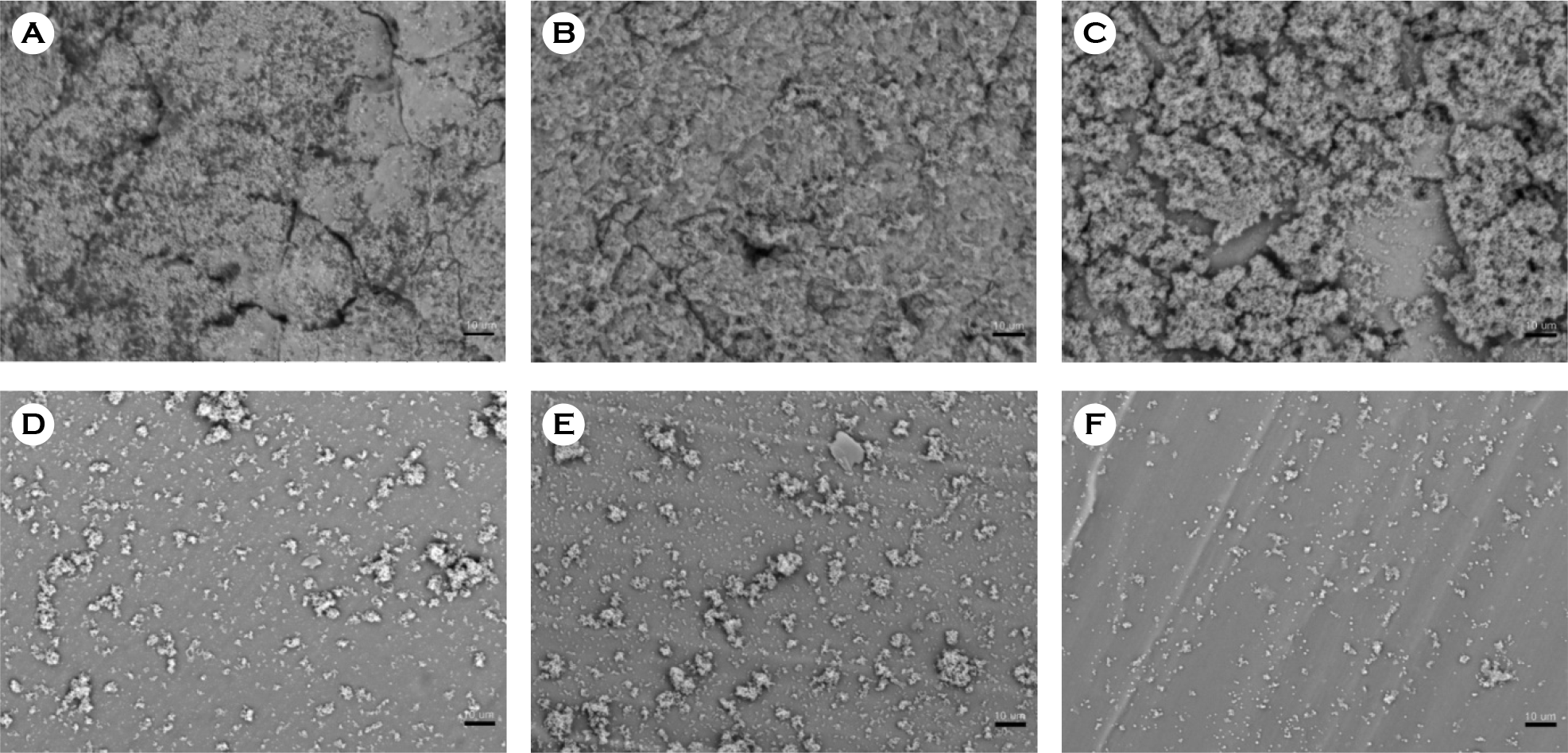 | Figure 5.Scanning electron micrographs of biofilm formation by S. aureus on catheter discs. (A) Latex, (B) anti-infective latex (IC-Latex), (C) silicone-elastomer coated latex (SEC), (D) 100% silicone, (E) hydrogel-coated silicone (HCS), (F) silver-alloy-coated silicone (SCS). (scale bar, 10 μm) |
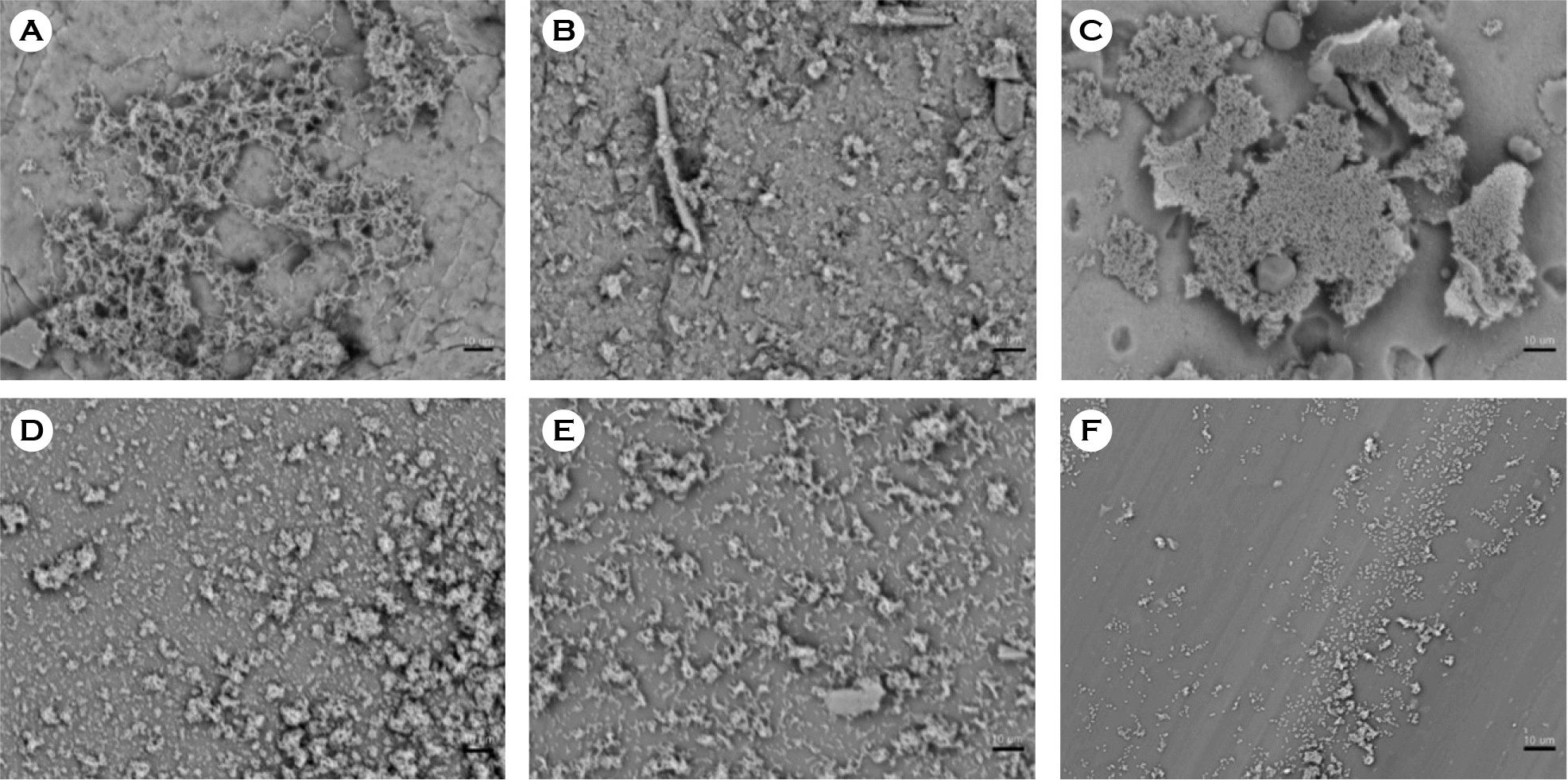 | Figure 6.Scanning electron micrographs of biofilm formation by S. salivarius on catheter discs. (A) Latex, (B) anti-infective latex (IC-Latex), (C) silicone-elastomer coated latex (SEC), (D) 100% silicone, (E) hydrogel-coated silicone (HCS), (F) silver-alloy-coated silicone (SCS). (scale bar, 10 μm) |
Table 1.
Biofilm formation ability of microorganisms on different catheter materials and cell surface hydrophobicity of microorganisms
| Species | Catheter materials | CSH (%) | |||||
|---|---|---|---|---|---|---|---|
| Latex | IC-Latex | SEC | Silicone | HCS | SCS | ||
| C. albicans | 2.09±0.039a | 1.39±0.055 | 2.913±0.180 | 2.105±0.020 | 1.3±0.139 | 0.83±0.02 | 58±1b |
| E. coli | 0.462±0.067 | 0.35±0.03 | 0.617±0.085 | 0.416±0.014 | 0.221±0.023 | 0.163±0.051 | 86±0 |
| P. vulgaris | 0.59±0.162 | 0.387±0.094 | 0.612±0.005 | 0.486±0.08 | 0.379±0.02 | 0.235±0.053 | 76±0 |
| S. aureus | 0.783±0.155 | 0.647±0.057 | 0.858±0.013 | 0.699±0.080 | 0.579±0.049 | 0.390±0.081 | 99±3.7 |
| S. salivarius | 1.116±0.105 | 0.772±0.061 | 1.324±0.084 | 0.706±0.016 | 0.576±0.022 | 0.297±0.002 | 98±1.6 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download