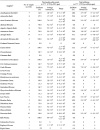
Based on the results from aerobic plate counts, samples with higher average aerobic bacteria counts were
Acanthopanax Root Bark,
Acori Gramineri Rhizoma,
Atractylodis Rhizoma Alba,
Cinnamoni Cortex Spissus,
Lyriopis Tuber, and
Lycii Fructus. Coliforms were prevalent in
Angelica Gigantis Radix and
Atractylodis Rhizoma Alba (
Table 1). These results were similar to the findings from Lee et al.'s report (
6), in which the following was observed: the total aerobic plate counts were smaller in
Astragali Radix,
Eucommiae Cortex, and
Zizyphi Semen, but much was measured in
Acori Gramineri Rhizoma,
Atractylodis Rhizoma Alba,
Achyranthis Radix, and
Liriopis Tuber.
Out of 194 agricultural herbal products, 491 bacteria were isolated. Out of these number, there was 51.3% (252/491) coliforms, 30.1% (148/491)
Bacillus spp., 15.3% (75/491)
Enterococcus spp., 2.0% (10/491)
Staphylococcus spp., and 1.2% (6/491)
Listeria spp. (
Table 2,
Table 3). Of them,
B. cereus was the most prevalent pathogenic species in the genus
Bacillus, but the most prevalent species in the genus
Bacillus was
B. mycoides (39.9%, 59/148) (
Table 2,
Table 3). This result is comparable with the results obtained by Oyetayo (
10). He assessed Nigerian herbal medicines and reported that almost all of the herbal medicines tested were contaminated with
Bacillus spp. which is commonly found in soil, air, dust, etc. (
10). Many aerobic species of
Bacillus spp. produce endospore that helps them not only resist environmental stress but also ensure their long-term survival under adverse conditions (
10). In his study, all agricultural herbal products in which microorganisms were not isolated in aerobic bacteria count showed an antibacterial property (
10). The antibacterial property of agricultural herbal products might be one of the reasons that they are not contaminated by bacteria, with the exception of
Bacillus spp. (
10). Among the
Bacillus spp., the most predominant species was
B. cereus (23.0%, 34/148) (
Table 2). Esimone et al. (
11) had also reported
Bacillus spp. (28.4%) as the most common organism in herbal products. The presence of
B. subtilis and
B. cereus observed in agricultural herbal products is comparable to a similar study conducted by Adeleye et al. (
12). Microbial contamination usually occurs due to improper drying or storage of the plant material which eventually results in degradation of the plant constituents (
4). Microbial contamination can also render plant material toxic, either by transforming the chemicals in the plant material or from the production of toxic compounds by the microbes (
4). Therefore, microbial quality tests should be conducted as necessary throughout the lifestyle of the plant materials, from the raw material to the finished product stages (
4). The result is comparable with the result obtained by Okunlola et al. (
13). They had also reported the presence of
Escherichia coli,
Salmonella spp. and
Staphylococcus aureus in agricultural herbal products. Similarly, the same microorganisms were also detected in this research. During the quality analysis, precautions must be taken to ensure that conditions do not adversely affect any microorganisms that are to be measured (
4).




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download