Abstract
The skin functions as a physical barrier against entry of pathogens while concomitantly supporting a myriad of commensal organisms. The characterization of these microbial communities has enhanced our knowledge of the ecology of organisms present in normal skin, and studies have begun to illuminate the intimate relationship between the host and resident microbes. The cutaneous innate and adaptive immune responses can modulate skin microbiota, while simultaneously, the microbiota educates the host immune system. A crucial element of the innate immune response is mast cells, which reside strategically in tissues that are commonly exposed to the external environment, such as the skin and mucosae. Mast cells are present on the frontline of defense against pathogens, suggesting they may play an important role in fostering the host-microbiota relationship. In this review, we highlight findings regarding the interaction between skin microbiota and mast cells and the resulting outcomes in skin homeostasis.
Go to : 
REFERENCES
1). Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seité S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp Dermatol. 2017; 26:798–803.

2). Takemoto A, Cho O, Morohoshi Y, Sugita T, Muto M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J Dermatol. 2015; 42:166–70.

3). Nada HA, Gomaa NI, Elakhras A, Wasfy R, Baker RA. Skin colonization by superantigen-producing Staphylococcus aureus in Egyptian patients with atopic dermatitis and its relation to disease severity and serum interleukin-4 level. Int J Infect Dis. 2012; 16:e29–33.

4). Csaba G. Mast cell, the peculiar member of the immune system: A homeostatic aspect. Acta Microbiol Immunol Hung. 2015; 62:207–31.

5). Forsythe P, Bienenstock J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy. 2012; 98:196–221.

6). Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010; 130:192–200.

7). Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, et al. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One. 2010; 5:e8557.
8). Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010; 130:2211–21.
9). Afrin LB, Khoruts A. Mast Cell Activation Disease and Microbiotic Interactions. Clin Ther. 2015; 37:941–53.

10). Wang Z, Mascarenhas N, Eckmann L, Miyamoto Y, Sun X, Kawakami T, et al. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J Allergy Clin Immunol. 2017; 139:1205–16.

11). Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008; 158:442–55.
12). Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013; 4:1431.

13). Wanke I, Steffen H, Christ C, Krismer B, Götz F, Peschel A, et al. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Invest Dermatol. 2011; 131:382–90.

14). Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011; 332:974–7.

15). Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008; 455:808–12.

16). Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008; 29:637–49.

17). Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003; 170:2274–8.

18). Wang Z, MacLeod DT, Di Nardo A. Commensal bacteria lipoteichoic acid increases skin mast cell antimicrobial activity against vaccinia viruses. J Immunol. 2012; 189:1551–8.

19). Leung AD, Schiltz AM, Hall CF, Liu AH. Severe atopic dermatitis is associated with a high burden of environmental Staphylococcus aureus. Clin Exp Allergy. 2008; 38:789–93.

20). Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017; 9.

21). Matsui K, Nishikawa A. Percutaneous application of peptidoglycan from Staphylococcus aureus induces an increase in mast cell numbers in the dermis of mice. Clin Exp Allergy. 2005; 35:382–7.

22). Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Muñoz-Planillo R, Hasegawa M, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013; 503:397–401.
23). Selander C, Zargari A, Möllby R, Rasool O, Scheynius A. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy. 2006; 61:1002–8.

24). Selander C, Engblom C, Nilsson G, Scheynius A, Andersson CL. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J Immunol. 2009; 182:4208–16.

25). Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014; 32:227–55.

26). Picciani BL, Michalski-Santos B, Carneiro S, Sampaio AL, Avelleira JC, Azulay DR, et al. Oral candidiasis in patients with psoriasis: correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. J Am Acad Dermatol. 2013; 68:986–91.

27). Taheri Sarvtin M, Shokohi T, Hajheydari Z, Yazdani J, Hedayati MT. Evaluation of candidal colonization and specific humoral responses against Candida albicans in patients with psoriasis. Int J Dermatol. 2014; 53:e555–60.
Go to : 
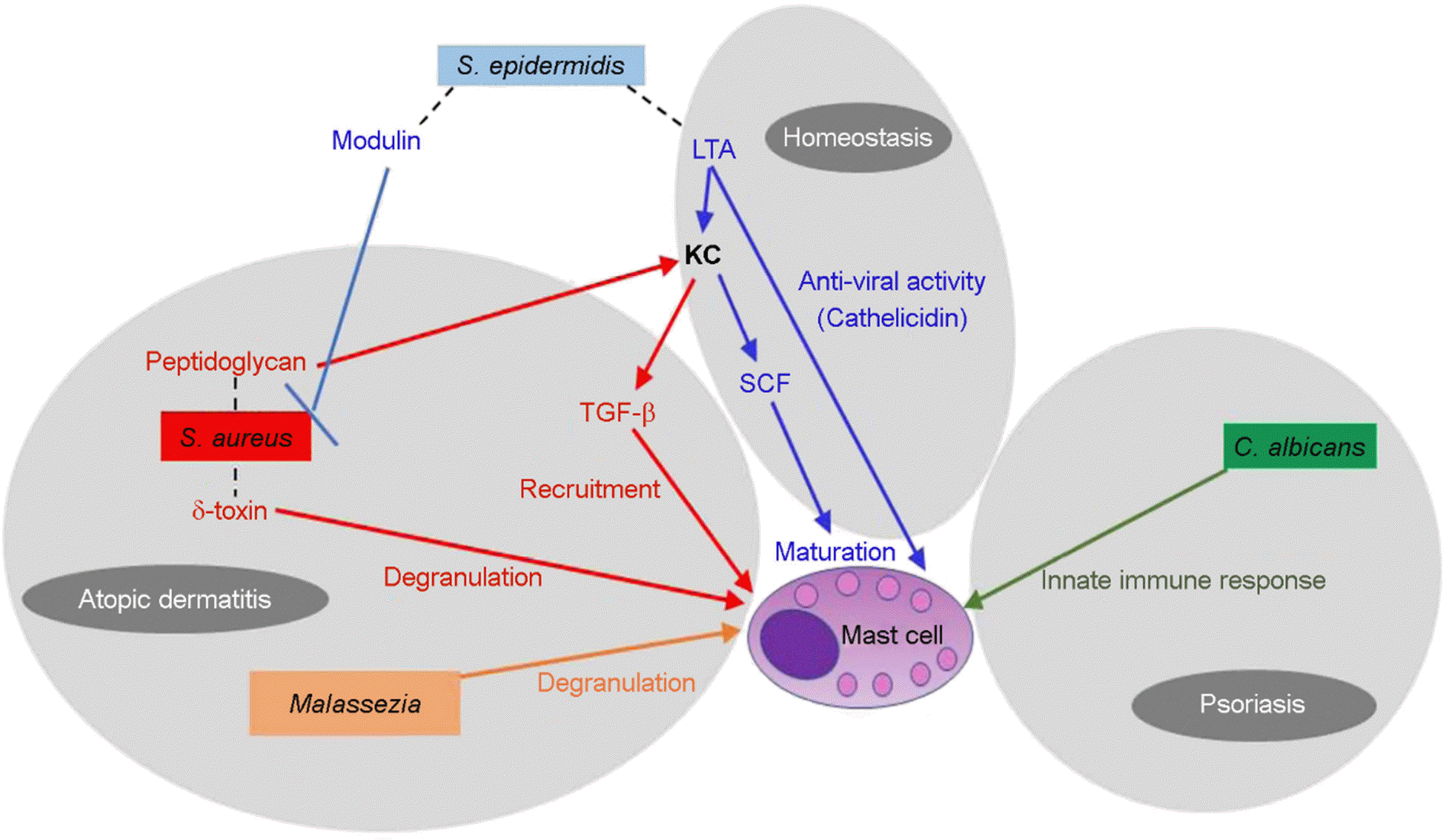 | Figure 1.The network in skin microbiome and mast cells. S. epidermidis-derived LTA reinforces the clearance of virus (e.g., vaccinia virus) by increasing antimicrobial peptide, cathelicidin, in mast cells. LTA also promote mast cell maturation by triggering SCF in KC. S. aureus derived peptidoglycan can stimulate KC to produce TGF-β which lead to mast cells recruitment to the dermis in atopic dermatitis. The other byproduct of S. aureus, δ-toxin directly induce mast cells degranulation in the pathogenesis of atopic dermatitis. Phenol-soluble modulins produced by S. epidermidis can selectively inhibit S. aureus. The lipophilic yeast, Malassezia sympodialis can activate mast cells and exacerbate the inflammatory response in atopic dermatitis. C. albicans is frequently presented in psoriasis patients and correlate with disease severity. Mast cells produce cytokines, chemokine, and ROS in response to C. albicans. LTA, lipoteichoic acid; KC, keratinocytes; SCF, stem cell factor; TGF-β, transforming growth factor beta |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download