Abstract
During recent decades, several times of frequent appearance of the “newly emerging or re-emerging” pathogens are posing a great threat to the human populations as well as many other animals. It is well known that most risky groups are viral pathogens. Among them, taking a pose a striking threat to the human is belong to the influenza A viruses (IAVs). Influenza or flu is a wide spread zoonotic disease caused by influenza A virus, which has their ultimate origin from avian reservoirs and may stably adapt, either directly or after several passages thru another mammalian species of host, to human populations. Novel human-adapted IAVs have emerged to cause pandemics several times in the last 100 years. Typical avian influenza A viruses are restricted from replicating efficiently and causing disease in humans. Mechanisms by which viruses evolve in one host, cause host switch, and adapt to a new host species from wild aquatic waterfowl to mammalian species including human, remain to be elucidated. Here, some insights into the mechanisms of IAV's host switch and their adaptation and viral virulence factors associated with a novel virus in human are briefly reviewed.
Go to : 
REFERENCES
1). Guan Y, Smith GJ. The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res. 2013; 178:35–43.

2). WHO. H5N1 avian influenza: Timeline of major events. Available at. http://www.who.int/influenza.
3). Empress-watch. FAO Avian influenza A(H5N6): the latest addition to emerging zoonotic avian influenza threats in East and Southeast Asia. 2014; 30:1–6.
4). Li C, Li C, Zhang AJ, To KK, Lee AC, Zhu H, et al. Avian influenza AH7N9 virus induces severe pneumonia in mice without prior adaptation and responds to a combination of Zanamivir and COX-2 inhibitor. PloS One. 2014; 9:e107966.
5). Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012; 336:1534–41.

6). Cauldwell AV, Long JS, Moncorgé O, Barclay WS. Viral determinants of influenza A virus host range. J Gen Virol. 2014; 95:1193–210.

7). Dugan VG, Chen R, Spiro DJ, Sengamakay N, Zaborsky J, Ghedin E, et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008; 4:e1000076.

8). Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–79.

9). Swayne DE. Understanding the complex pathobiology of high pathogenecity avian influenza viruses in birds. Avian Dis. 2007; 51:242–9.
10). Reperant LA, Rimmelzwaan GF, Kuiken T. Avian influenza viruses in mammals. Rev Sci Tech. 2009; 28:137–59.

11). Joseph U, Su YC, Vijaykrisna D, Smith GJ. The ecology and adaptive evolution of influenza A interspecies transmission. Influenza Other Respir Viruses. 2017; 11:74–84.

12). Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004; 101:1356–61.

13). Skowronski DM, Tweed SA, Petric M, Booth T, Li Y, Tam T. Human illness and isolation of low-pathogenicity avian influenza virus of the H7N3 subtype in British Columbia, Canada. J Infect Dis. 2006; 193:899–900.

14). WHO. cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. 2011.
15). Kuiken T, Holmes EC, McCauley J, Rimmelzwaan GF, Williams CS, Grenfell BT. Host species barriers to influenza virus infections. Science. 2006; 312:394–7.

16). Taubenberger JK, Morens DM. Pandemic influenza-including a risk assessment of H5N1. Rev Sci Tech. 2009; 28:187–202.
17). Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott, Williams & Wilkins;2007. p. 1691–740.
18). Earn DJ, Dushoff J, Levin SA. Ecology and evolution of the flu. Trends Ecol Evol. 2002; 17:334–40.

19). Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009; 459:931–9.

20). Van Riel D, den Bakker MA, Leijten LM, Chutinimitkul S, Munster VJ, de Wit E, et al. Seasonal and pandemic human influenza viruses attach better to human upper respiratory tract epithelium than avian influenza viruses. Am J Pathol. 2010; 176:1614–8.

21). Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006; 440:435–6.
22). Resa-Infante P, Jorba N, Coloma R, Ortin J. The influenza virus RNA synthesis machine: advances in its structure and function. RNA Biol. 2011; 8:207–15.
23). Perez DR, Webby RJ, Hoffmann E, Webster RG. Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis. 2003; 47:1114–7.
24). Campitelli L, Mogavero E, De Marco MA, Delogu M, Puzelli S, Frezza F, et al. Interspecies transmission of an H7N3 influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology. 2004; 323:24–36.

25). Wasilenko JL, Lee CW, Sarmento L, Spackman E, Kapczynski DR, Suarez DL, et al. NP, PB1, and PB2 viral genes contribute to altered replication of H5N1 avian influenza viruses in chickens. J Virol. 2008; 82:4544–53.

26). Blok J, Air GM. Variation in the membrane-insertion and "stalk" sequences in eight subtypes of influenza type A virus neuraminidase. Biochemistry. 1982; 21:4001–7.

27). Landolt GA, Olsen CW. Up to new tricks-a review of cross-species transmission of influenza A viruses. Anim Health Res Rev. 2007; 8:1–21.
28). Taubenberger JK, Reid AH, Janczewski TA, Fanning TG. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philos Trans R Soc Lond B Biol Sci. 2001; 356:1829–39.

29). Olsen CW. The emergence of novel swine influenza viruses in North America. Virus Res. 2002; 85:199–210.

30). Dunham EJ, Dugan VG, Kaser EK, Perkins SE, Brown IH, Holmes EC, et al. Different evolutionary trajectories of European avian-like and classical swine H1N1 influenza A viruses. J Virol. 2009; 83:5485–94.

31). Nicolls JM, Bourne AJ, Chen H, Guan Y, Peiris JS. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res. 2007; 8:73.

32). Horimoto T, Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clin Microbiol Rev. 2001; 14:129–49.

33). Qi L, Davis AS, Jagger BW, Schwartzman LM, Dunham EJ, Kash JC, et al. Analysis by single-gene reassortment demonstrates that the 1918 influenza virus is functionally compatible with a low-pathogenicity avian influenza virus in mice. J Virol. 2012; 86:9211–20.

34). Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008; 198:962–70.

35). Morens DM, Taubenberger JK, Fauci AS. The persistent legacy of the 1918 influenza virus. N Engl J Med. 2009; 361:225–9.

36). Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989; 63:4603–8.

37). Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009; 325:197–201.
38). Peiris JS, de Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007; 20:243–67.

39). Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, et al. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis. 2004; 10:2196–9.

40). Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, et al. Avian-to human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci U S A. 2000; 97:9654–8.
41). Claes F, Morzaria SP, Donis RO. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses-how is the Asian HPAI H5 lineage maintained. Curr Opin Virol. 2016; 16:158–63.
42). Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998; 279:393–6.

43). Yang L, Zhu W, Li X, Bo H, Zhang Y, Gao R, et al. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses. J Virol. 2016.

44). Chen R, Holmes EC. Avian influenza virus exhibits rapid evolutionary dynamics. Mol Biol Evol. 2006; 23:2336–41.

45). Claas EC, de Jong JC, van Beek R, Rimmelzwaan GF, Osterhaus AD. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998; 16:977–8.

47). Matrosovich MN, Krauss S, Webster RG. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology. 2001; 281:156–62.

48). Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007; 44:1084–8.

49). Shinde S, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, et al. Triple-reassortant swine influenza A (H1) in humans in the United states, 2005–2009. N Engl J Med. 2009; 360:2616–25.

50). Van Reeth K, Nicoll A. A human case of swine influenza virus infection in Europe-implications for human health and research. Euro Surveill. 2009; 14.

51). Rabadan R, Levine AJ, Robins H. Comparison of avian and human influenza A viruses reveals a mutational bias on the viral genomes. J Virol. 2006; 80:11887–91.

52). Zaraket H, Bridges OA, Russell CJ. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus replication and pathogenesis in mice. J Virol. 2013; 87:1826–34.

53). Matrosovich M, Stech J, Klenk HD. Influenza receptors, polymerase and host range. Rev Sci Tech. 2009; 28:203–17.

54). Qi L, Kash JC, Dugan VG, Wang R, Jin G, Cunningham RE, et al. Role of sialic acid binding specificity of the 1918 influenza virus hemagglutinin protein in virulence and pathogenesis for mice. J Virol. 2009; 83:3754–61.

55). Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solórzano A, Pappas C, et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007; 315:655–9.

56). Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006; 443:578–81.

57). Ayllon J, Russell RJ, Garcia-Sastre A, Hale BG. Contribution of NS1 effector domain dimerization to influenza A virus replication and virulence. J Virol. 2012; 86:13095–8.

58). Moncorgé O, Mura M, Barclay WS. Evidence for avian and human host cell factors that affect the activity of influenza virus polymerase. J Virol. 2010; 84:9978–86.

59). Ping J, Keleta I, Forbes NF, Dankar S, Stecho W, Tyler S, et al. Genomic and protein structural maps of adaptive evolution of human influenza A virus to increased virulence in the mouse. PLoS One. 2011; 6:e21740.

60). Hatta M, Hatta Y, Kim JH, Watanabe S, Shinya K, Nguyen T, et al. Growth of H5N1 influenza A viruses in the upper respiratory tract of mice. PLoS Pathog. 2007; 3:1374–9.
61). Cauldwell AV, Moncorgé O, Barclay WS. Unstable polymerasenucleoprotein interaction is not responsible for avian influenza virus polymerase restriction in human cells. J Virol. 2013; 87:1278–84.

62). Massin P, van der Werf S, Naffakh N. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J Virol. 2001; 75:5398–404.
63). Mehle A, Doudna JA. Adaptive strategies of the influenza virus polymerase for replication in humans. Proc Natl Acad Sci U S A. 2009; 106:21312–6.

64). Bortz E, Westera L, Maamary J, Steel J, Albrecht RA, Manicassamy B, et al. Hostand strain-specific regulation of influenza virus polymerase activity by interacting cellular proteins. MBio. 2011; 2:e00151–11.

65). Xu C, Hu WB, Xu K, He YX, Wang TY, Chen Z, et al. Amino acids 473V and 598P of PB1 from an avian-origin influenza A virus contribute to polymerase activity, especially in mammalian cells. J Gen Virol. 2012; 93:531–40.

66). Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Zaslavsky L, Tatusova T, et al. The influenza virus resource at the National Center for Biotechnology Information. J Virol. 2008; 82:596–601.

67). Gabriel G, Dauber B, Wolff T, Planz O, Klenk HD, Stech J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc Natl Acad Sci U S A. 2005; 102:18590–5.

68). Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marijuki H, et al. Adaptation of pandemic H1N1 influenza viruses in mice. J Virol. 2010; 84:8607–16.

69). Mänz B, Brunotte L, Reuther P, Schwemmle M. Adaptive mutations in NEP compensate for defective H5N1 RNA replication in cultured human cells. Nat Commun. 2012; 3:802.

70). Mänz B, Schwemmle M, Brunotte L. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J Virol. 2013; 87:7200–9.
Go to : 
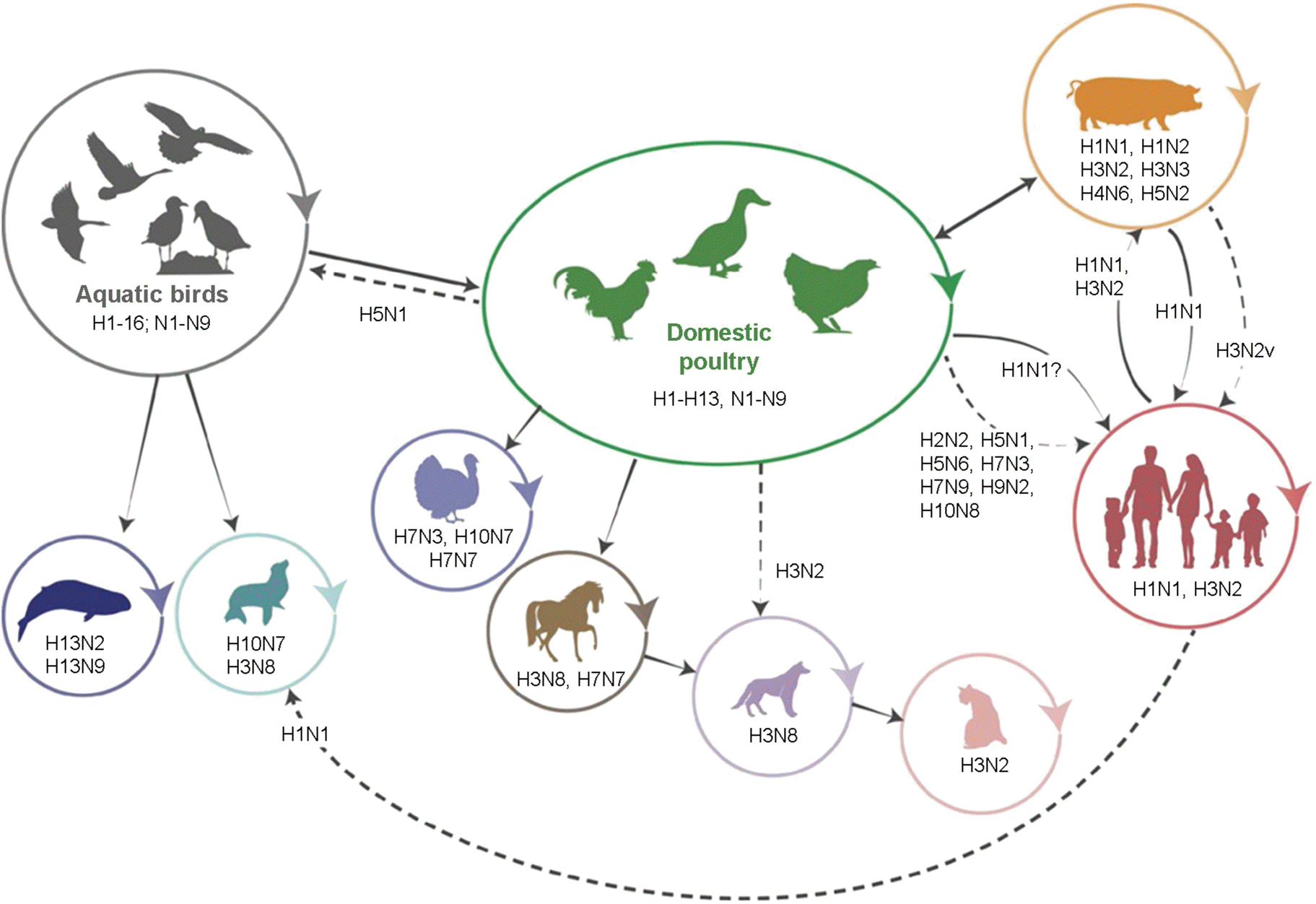 | Figure 1.Influenza A viruses (IAVs) transmission between interspecies. Representative diagram of interspecies transmission and the subtypes in these events. Solid arrow shows direct transmission and dashed arrows represent sporadic or limited infection of subtypes (11). |
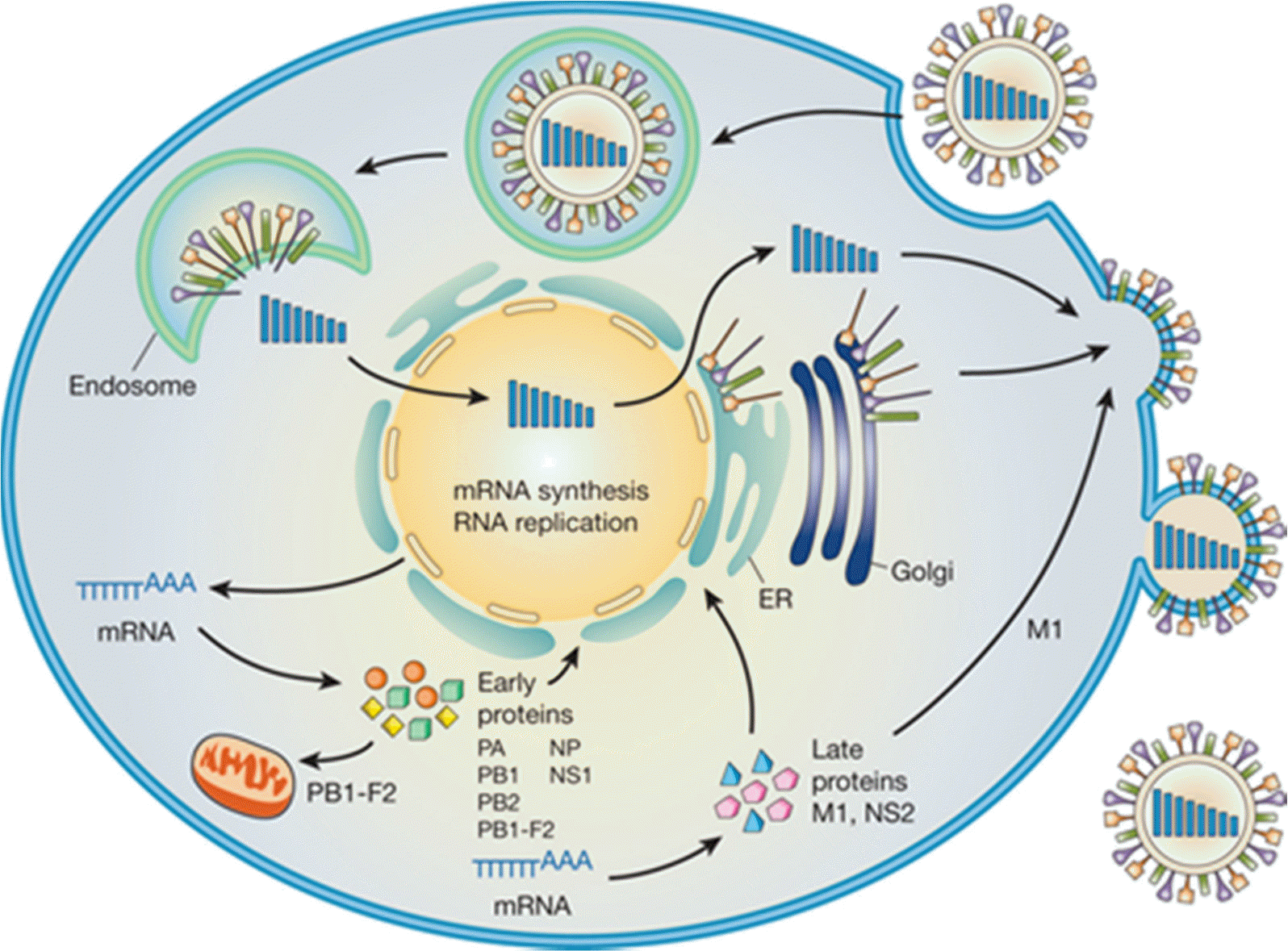 | Figure 2.Schematic diagram of the influenza viral life cycle. Following receptor-mediated endocytosis, vRNP complexes are released into the cytoplasm and subsequently transported to the nucleus, where replication and transcription take place. mRNAs are exported to the cytoplasm for translation. Early proteins are transported back to the nucleus for replication and transcrition. Late proteins are used for the vRNPs. The assembly and budding of progeny virions occurs at the plasma membrane (19). |
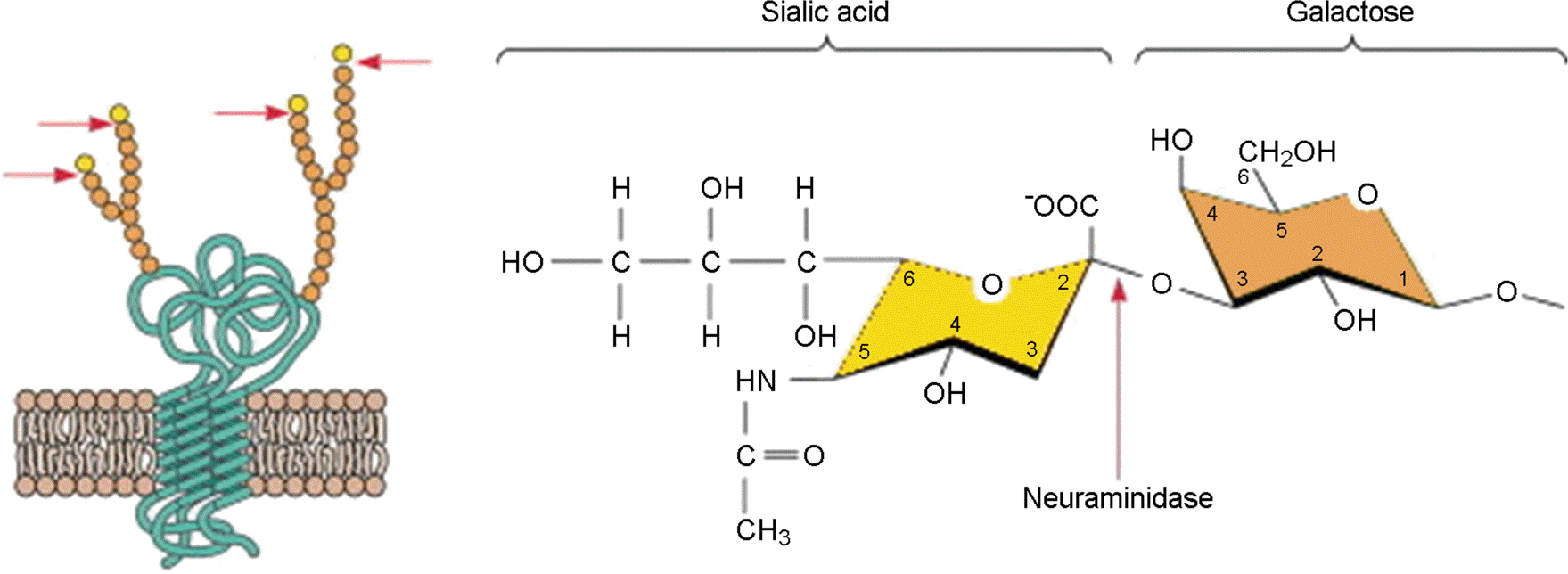 | Figure 3.A structural model of the influenza virus receptor molecule on the surface of the host cell. (left) A sialic acid molecule is always attached to the second glyco-molecule of the galactose in the series of polysaccharides which is attached to the receptor proteins embedded into cytoplasmic membrane. (right) Sialic acid and nascent galactose sugar are linked with α2,3-linkage at the surface of human cell, meanwhile avian virus is specifically attached to the α2,6-linkage between sialic acid and galactose. NA proteins on the surface of virion cleave these linkage when they are released form an infected cell surface. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download