Abstract
Getah virus (GETV) is a member of the genus Alphavirus in the family Togaviridae. GETV infection can occur in a wide range of vertebrate species, and the virus has been known for a pathogen of horses and pigs. To rapidly and accurately diagnose GETV infection of a racehorse, an indirect ELISA (I-ELISA) was developed in the present study for detection of antibodies to GETV in serum samples. To evaluate the developed I-ELISA, a total of 240 serum samples from Thoroughbred racehorses raised in Korea were screened in parallel by a serum neutralization (SN) test. The developed I-ELISA exhibited an efficacy comparable to that of the SN test in terms of a high diagnostic sensitivity (86.3%) and specificity (94.5%) at a cut-off absorbance value of 0.25. In addition, our results showed that the developed I-ELISA had a significant correlation with the SN test (r = 0.91; p < 0.05). Taken together, our findings suggest that the I-ELISA developed in this study is a valuable diagnostic tool for the screening of horses suspected to be infected with GETV.
Go to : 
REFERENCES
1). Berge TO. International catalogue of arboviruses, including certain other viruses of vertebrates. In: DHEW Publication No. (CDC) 78-8301. Washington D.C.: US Department of Health, Education and Welfare, Public Health Service;. 1975. 278.
2). Calisher CH, Karabatsos N. Arbovirus serogroups: definition and geographic distribution. The Arboviruses: Epidemiology and Ecology. Monath TP, editor. I. Boca Raton, FL: CRC Press;1988. p. 19–57.

3). Weaver SC, Dalgarno L, Frey TK, Huang HV, Kinney RM, Rice CM, et al. Family Togaviridae. Virus Taxonomy: Seventh Report of the International Committee on Taxonomy of Viruses. van Regenmortel MV, Fauguet CM, Bishop DHI, Carstens EB, Estes MK, Lemon SM, editors. San Diego: Academic Press;2000. p. 879–89.
4). Bryant JE, Crabtree MB, Nam VS, Yen NT, Duc HM, Miller BR. Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am J Trop Med Hyg. 2005; 73:470–3.

5). Chang CY, Huang CC, Huang TS, Deng MC, Jong MH, Wang FI. Isolation and characterization of a Sagiyama virus from domestic pigs. J Vet Diagn Invest. 2006; 18:156–61.

6). Norder H, Lundström JO, Kozuch O, Magnius LO. Genetic relatedness of Sindbis virus strains from Europe, Middle East, and Africa. Virology. 1996; 222:440–5.

7). Turell MJ, O'Guinn ML, Wasieloski LP Jr, Dohm DJ, Lee WJ, Cho HW, et al. Isolation of Japanese encephalitis and Getah viruses from mosquitoes (Diptera: Culicidae) collected near Camp Greaves, Gyonggi Province, Republic of Korea, 2000. J Med Entomol. 2003; 40:580–4.
8). Doherty RL, Gorman BM, Whitehead RH, Carley JG. Studies of arthropod-borne virus infections in Queensland: V. Survey of antibodies to Group A arboviruses in man and other animals. Aust J Exp Biol Med Sci. 1966; 44:365–77.
9). Go YY, Balasuriya UB, Lee CK. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014; 3:58–77.

10). Fukunaga Y, Kumanomido T, Kamada M. Getah virus as an equine pathogen. Vet Clin North Am Equine Pract. 2000; 16:605–17.

11). Calisher CH, Walton TE. Getah virus infections. Virus Infections of Equines. Studdert MK, editor. Amsterdam: Elsevier;1996. p. 157–65.
12). Sentsui H, Kono Y. An epidemic of Getah virus infection among racehorses: Isolation of the virus. Res Vet Sci. 1980; 29:157–61.

13). Kumanomido T, Kamada M, Wada R, Kenemaru T, Sugiura T, Akiyama Y. Pathogenicity for horses of original Sagiyama virus, a member of the Getah virus group. Vet Microbiol. 1988; 17:367–73.

14). Kumanomido T, Wada R, Kanemaru T, Kamada M, Hirasawa K, Akiyama Y. Clinical and virological observations on swine experimentally infected with getah virus. Vet Microbiol. 1988; 16:295–301.

15). Brown CM, Timoney PJ. Getah virus infection in Indian horses. Trop Anim Hlth Prod. 1988; 30:241–52.
16). Timoney PJ. Getah virus infection. Infectious Diseases of Livestock. Coetzer JAW, Tustin RC, editors. 2nd ed.Cape Town: Oxford University Press;2004. p. 1023–6.
17). Mair TS, Timoney PJ. Getah virus infection. Infectious Disease of the Horse. Mair TS, editor. Ashford: Geerings Print Ltd;2009. p. 155–8.
18). Imagawa H, Ando Y, Kamada M, Sugiura T, Kumanomido T, Fukunaga Y, et al. Seroepizootiological survey of Getah virus infection in light horses in Japan. Nihon Juigaku Zasshi. 1981; 43:797–802.
19). Sugiura T, Shimada K. Seroepizootiological survey of Japanese encephalitis virus and Getah virus in regional horse race tracks from 1991 to 1997 in Japan. J Vet Med Sci. 1999; 61:877–81.

20). Kamada M, Kumanomido T, Wada R, Fukunaga Y, Imagawa H, Sugiura T. Intranasal infection of Getah virus in experimental horses. J Vet Med Sci. 1991; 53:855–8.

21). Shortridge KF, Mason DK, Watkins KL, Aaskov JG. Serological evidence for the transmission of Getah virus in Hong Kong. Vet Rec. 1994; 134:527–8.

22). Jo HY, Yang DK, Kim HH, Choi SS, Kang KS, Yang SJ, et al. Sero-surveillance of Getah virus among Thoroughbred horses in Korea. J Bacteriol Virol. 2015; 45:225–41.

23). Fukunaga Y, Kumanomido T, Imagawa H, Ando Y, Kamada M, Wada R, et al. Isolation of picornavirus from horses associated with Getah virus infection. Nihon Juigaku Zasshi. 1981; 43:569–72.

24). Kamada M, Ando Y, Fukunaga Y, Kumanomido T, Imagawa H, Wada R, et al. Equine Getah virus infection: Isolation of the virus from racehorses during an enzootic in Japan. Am J Trop Med Hyg. 1980; 29:984–8.

25). Kumar J, Khan M, Gupta G, Bhoopati M, Lakshmana Rao PV, Parida M. Production, Characterization, and Application of Monoclonal Antibodies Specific to Recombinant (E2) Structural Protein in Antigen-Capture ELISA for Clinical Diagnosis of Chikungunya Virus. Viral Immunol. 2012; 25:153–60.
26). Chanas AC, Johnson KB, Simpson DIH. A comparative study of related alphaviruses-a naturally occurring model of antigenic variation in the Getah sub-group. J Gen Virol. 1977; 35:455–62.
27). Hohdatsu T, Ide S, Yamagishi H, Eiguchi Y, Nagano H, Maehara N, et al. Enzyme-linked immunosorbent assay for the serological survey of Getah virus in pigs. Nihon Juigaku Zasshi. 1990; 52:835–7.

28). Lee SH, Yang DK, Kim HH, Jo HY, Choi SS, Park JW, et al. Recharacterization of morphological and genetic feature of Getah virus isolated from South Korea. J Bacteriol Virol. 2015; 45:328–38.

29). Yang DK, Kim BH, Lim SI, Kwon JH, Lee KW, Choi CU, et al. Development and evaluation of indirect ELISA for the detection of antibodies against Japanese encephalitis virus in swine. J Vet Sci. 2006; 7:271–5.

30). Colombet J, Sime-Ngando T. Use of PEG, Polyethylene glycol, to characterize the diversity of environmental viruses. Microbial Ecology. 2012; 58:728–36.
31). Vajda BP. Concentration and purification of viruses and bacteriophages with polyethylene glycol. Folia Microbiol (Praha). 1978; 23:88–96.

32). KOVAØÈÍK K. The development and application of an indirect ELISA test for the detection of antibodies to bovine respiratory syncytial virus in blood serum. Vet Med-Czech. 2001; 46:29–34.
Go to : 
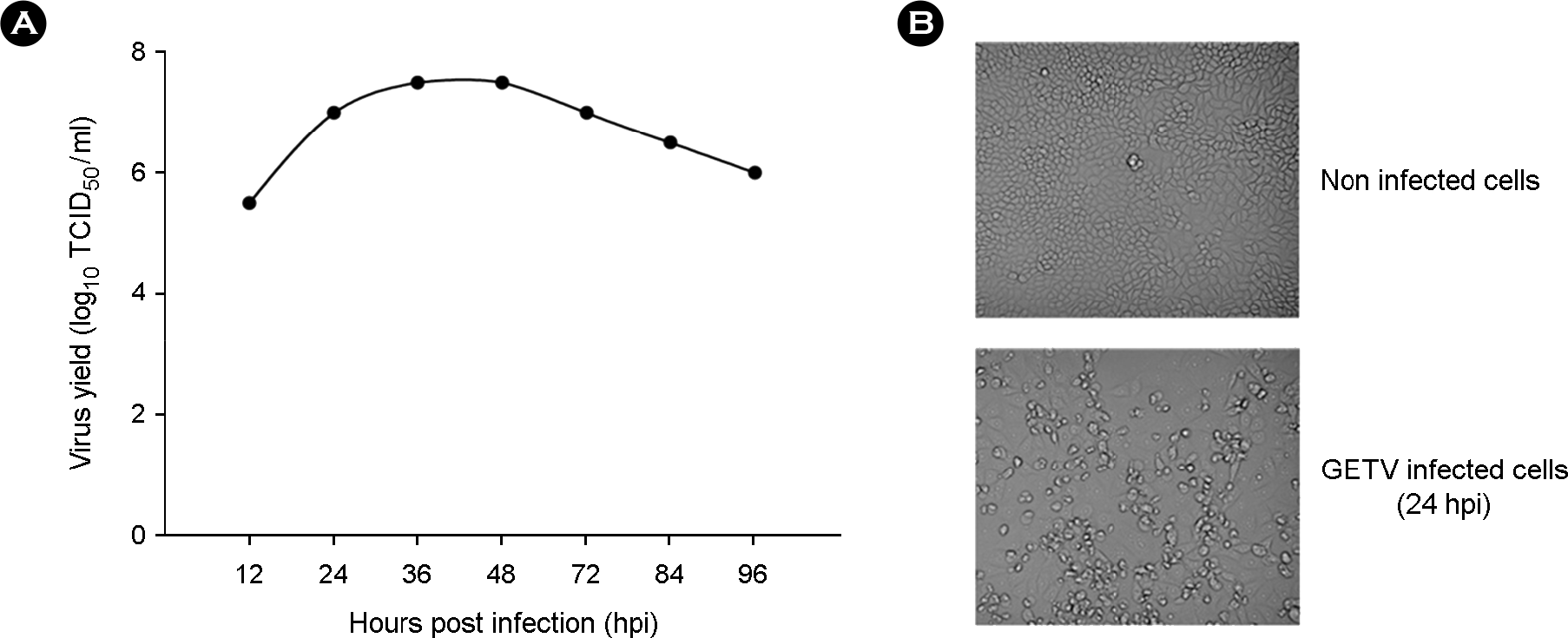 | Figure 1.The optimal time point for antigen harvest was determined by assessing GETV growth kinetics (A). The GETV-specific CPE was observed at ~24 hpi (B). |
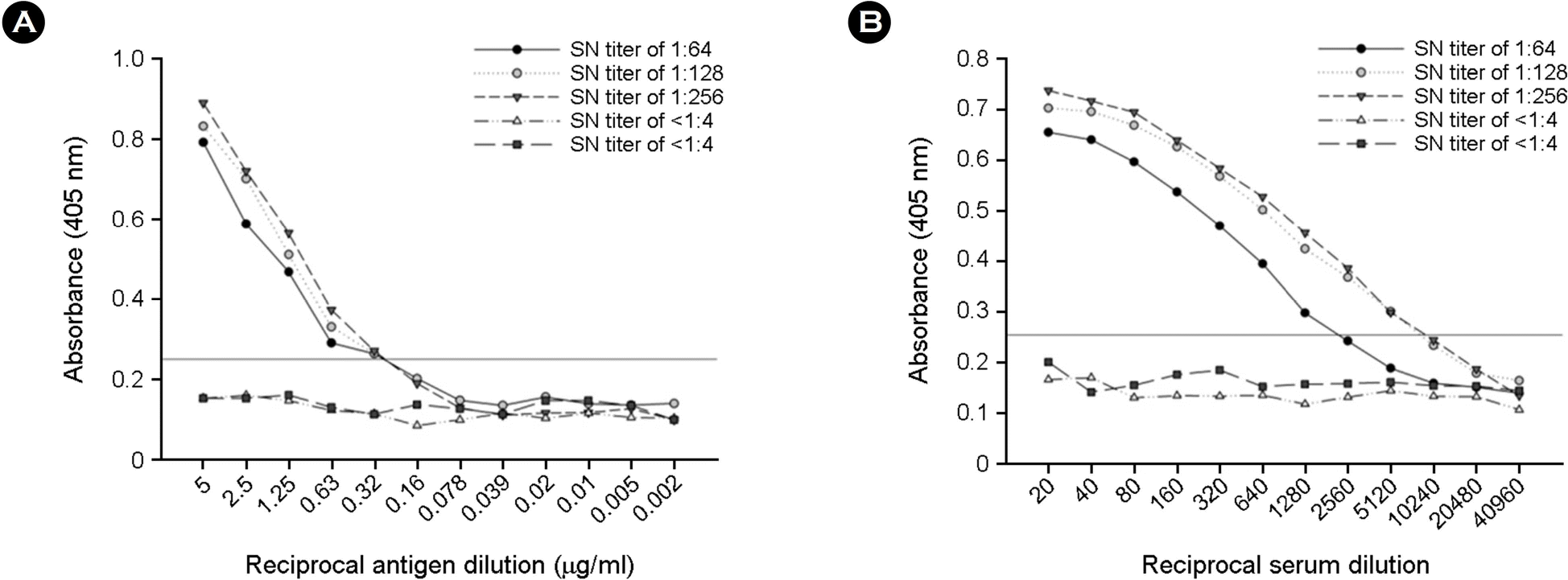 | Figure 2.Checkerboard titration of horse serum against GETV antigen by I-ELISA. GETV-positive (SN titer of ≥ 1:64) and -negative (SN titer of < 1:2) reference sera were determined by the SN test. According to the checkerboard titration, the optimum working concentration of GETV antigen (A) and dilution of reference sera (B) were 2.5 μg/ml and 1:40, respectively. The senum samples were evaluated as positive if their OD value was greater than 0.25. This OD cut-off value was calculated as noted in the Materials and Methods. |
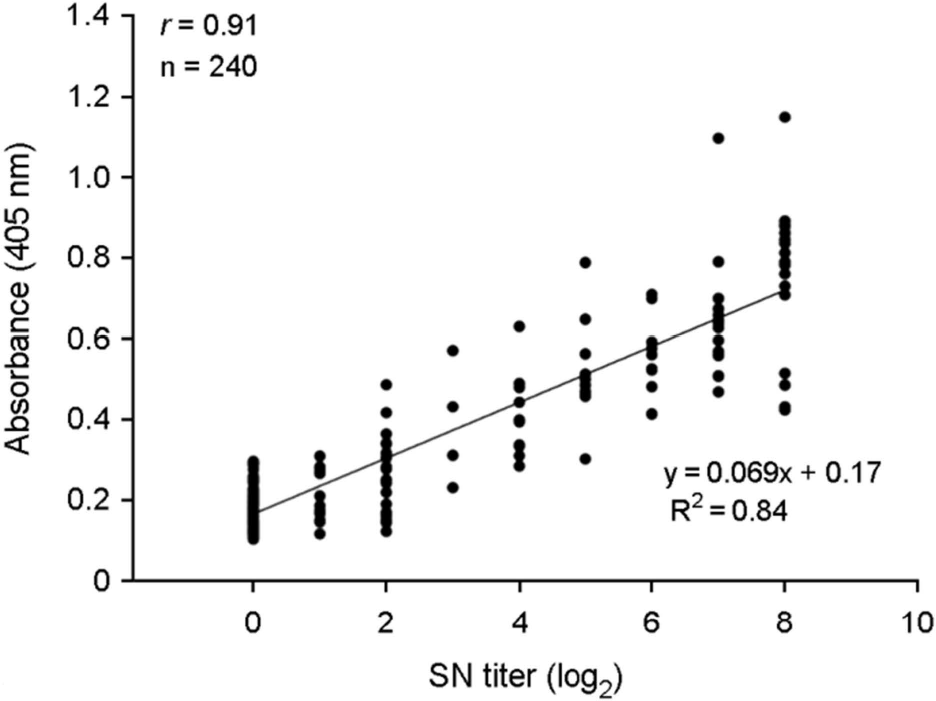 | Figure 3.Comparison of the SN test and I-ELlSA for detection of GETV antibodies in 240 horse serum samples. The correIation between the two tests is indicated by the linear regression line and r-value. |
Table 1.
Determination of the sensitivity and specificity of the I-ELISA for detection of GETV antibodies in comparison with the SN test. The diagnostic sensitivity and specificity of the I-ELISA test were 86.3% and 94.5%, respectivelya
| I-ELISA | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| SN test | Positive | 82 | 8 | 90 |
| Negative | 13 | 137 | 150 | |
| Total | 95 | 145 | 240 | |
| Overall agreement 91.25% | ||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download