Abstract
Mycobacterium tuberculosis (Mtb) causing tuberculosis as an intracellular pathogen initially infects alveolar macrophages following aerosol inhalation. Thus, macrophages play a critical role in the establishment of Mtb infection and macrophage cell death, a common outcome during Mtb infection, may initiate host- or pathogen-favored immune responses, resulting in facilitating protection or pathogenesis, respectively. In addition, virulent Mtb strains are known to inhibit apoptosis and consequently down-regulates immune response using a variety of strategies. In many recent studies have shown that virulent Mtb can either augment or reduce apoptosis by regulating expression of pro-apoptotic and anti-apoptotic proteins belonging to Bcl-2 family proteins. In this review, we will discuss and dissect the apoptotic pathways of Bcl-2 family proteins in Mtb-infected macrophages.
Go to : 
REFERENCES
1). Wipperman MF, Sampson NS, Thomas ST. Pathogen roid rage: cholesterol utilization by Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol. 2014; 49:269–93.
2). Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008; 205:2791–801.

3). Behar SM, Martin CJ, Booty MG, Nishimura T, Zhao X, Gan HX, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011; 4:279–87.
4). Abebe M, Kim L, Rook G, Aseffa A, Wassie L, Zewdie M, et al. Modulation of cell death by M. tuberculosis as a strategy for pathogen survival. Clin Dev Immunol. 2011; 2011:678570.
5). Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006; 176:3707–16.
6). Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, et al. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004; 172:6272–80.
7). Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004; 173:2660–8.
8). Parandhaman DK, Narayanan S. Cell death paradigms in the pathogenesis of Mycobacterium tuberculosis infection. Front Cell Infect Microbiol. 2014; 4:31.

9). Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996; 64:683–90.

10). Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol. 2003; 170:430–7.
11). Pedruzzi G, Das PN, Rao KV, Chatterjee S. Understanding PGE2, LXA4 and LTB4 balance during Mycobacterium tuberculosis infection through mathematical model. J Theor Biol. 2016; 389:159–70.
14). Aguiló N, Uranga S, Marinova D, Martin C, Pardo J. Bim is a crucial regulator of apoptosis induced by Mycobacterium tuberculosis. Cell Death Dis. 2014; 5:e1343.
15). Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003; 193:10–21.

16). Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007; 8:405–13.

17). Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008; 9:47–59.

18). Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009; 50:1–11.

19). Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014; 345:1250256.
20). Halder P, Kumar R, Jana K, Chakraborty S, Ghosh Z, Kundu M, et al. Gene expression profiling of Mycobacterium tuberculosis Lipoarabinomannan-treated macrophages: A role of the Bcl-2 family member A1 in inhibition of apoptosis in mycobacteria-infected macrophages. IUBMB Life. 2015; 67:726–36.
21). Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005; 73:1907–16.

22). Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000; 2:265–73.
23). Fratazzi C, Arbeit RD, Carini C, Balcewicz-Sablinska MK, Keane J, Kornfeld H, et al. Macrophage apoptosis in mycobacterial infections. J Leukoc Biol. 1999; 66:763–4.

24). Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000; 164:2016–20.
25). Gan H, Lee J, Ren F, Chen M, Kornfeld H, Remold HG. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat Immunol. 2008; 9:1189–97.
26). McGarvey JA, Wagner D, Bermudez LE. Differential gene expression in mononuclear phagocytes infected with pathogenic and non-pathogenic mycobacteria. Clin Exp Immunol. 2004; 136:490–500.

27). Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A. 2005; 102:8327–32.
28). Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007; 3:e110.
29). Aguilo JI, Alonso H, Uranga S, Marinova D, Arbués A, de Martino A, et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell Microbiol. 2013; 15:1994–2005.
30). Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000; 7:1182–91.

31). Garry RF. Extensive antigenic mimicry by retrovirus capsid proteins. AIDS Res Hum Retroviruses. 1990; 6:1361–2.

32). Zhou W, Hu J, Tang H, Wang D, Huang X, He C, et al. Small interfering RNA targeting mcl-1 enhances proteasome inhibitor-induced apoptosis in various solid malignant tumors. BMC Cancer. 2011; 11:485.

33). Mogga SJ, Mustafa T, Sviland L, Nilsen R. Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand J Immunol. 2002; 56:383–91.
34). Sohn H, Lee KS, Kim SY, Shin DM, Shin SJ, Jo EK, et al. Induction of cell death in human macrophages by a highly virulent Korean Isolate of Mycobacterium tuberculosis and the virulent strain H37Rv. Scand J Immunol. 2009; 69:43–50.
35). Wang FY, Wang XM, Wang C, Wang XF, Zhang YQ, Wu JD, et al. Suppression of Mcl-1 induces apoptosis in mouse peritoneal macrophages infected with Mycobacterium tuberculosis. Microbiol Immunol. 2016; 60:215–27.
Go to : 
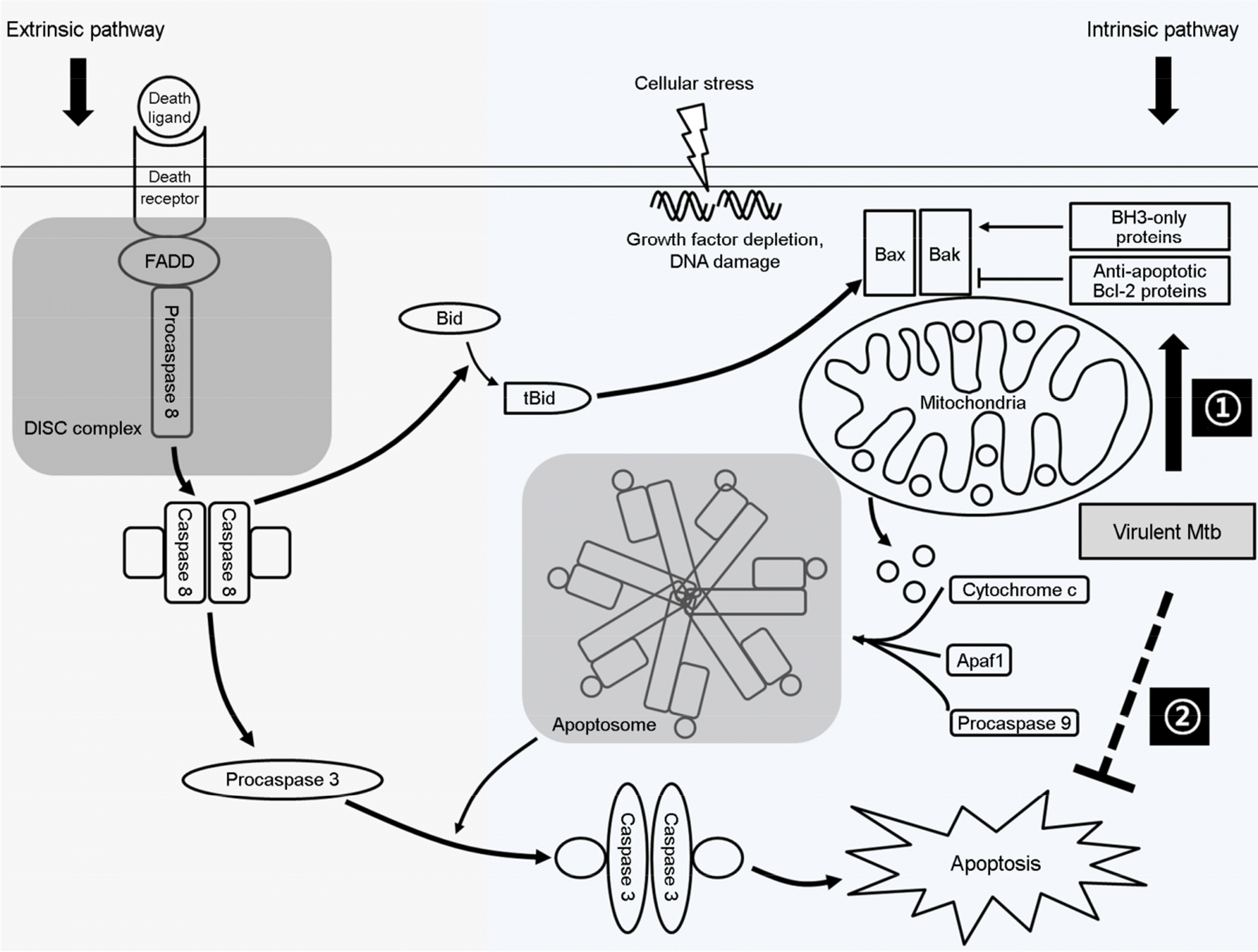 | Figure 1.Regulation of apoptosis signaling pathway by Bcl-2 family proteins. The mitochondrial outer membrane permeabilization (MOMP) can be regulated by either extrinsic (death receptor-mediated) and intrinsic (mitochondria-mediated) pathways. MOMP triggers the release of apoptogenic factor such as cytochrome c into cytosol to facilitate activation of caspase 3, resulting in induction of apoptosis. Virulent Mtb promotes the induction and production of Bcl-2 family proteins (①) and eventually inhibits apoptosis pathways (②) to multiplicate inside macrophages. FADD; Fas-associated death domain, Bax; Bcl-2-associated X protein, Bak; Bcl-2-antagonist killer, (t)Bid;(truncated) Bax-like BH3 protein, DISC; Death inducing signaling complex, Apaf1; Apoptotic protease activating factor 1, Mtb; Mycobacterium tuberculosis
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download