Abstract
In the previous study, we found that flavonoids and ginsenosides exhibited high eliminate rates of human immunodeficiency virus type 1 (HIV-1) D3-transfected macrophages. Based on these findings, here we synthesized the derivatives of gallic acid, including methyl gallate, methyl 4-O-methyl gallate, methyl 3,4-O-dimethyl gallate, and methyl 3,4,5-O-trimethyl gallate and measured their cellular toxic effects against HIV-1-infected macrophages. Of these, treatment with methyl 4-O-methyl gallate in the presence of lipopolysaccharide (LPS) and cycloheximide (CHX) most effectively eliminated HIV-1-transfected cytoprotective human microglial CHME5 cells and HIV-1-D3-infected human primary macrophages. Furthermore, these strongly inhibited LPS/CHX-induced phosphorylation of phosphoinositide 3-kinase (PI3K), pyruvate dehydrogenase lipoamide kinase isozyme 1 (PDK1), Akt, and glycogen synthase kinase-3β (GSK-3β) in the Tat-transfected cells and HIV-1-D3-infected human primary macrophages. These findings suggest that methyl 4-O-methyl gallate may be a promising candidate for eliminating HIV-1 infected macrophages by blocking PI3K/Akt signaling pathway.
Go to : 
REFERENCES
1). Chugh P, Bradel-Tretheway B, Monteiro-Filho CM, Planelles V, Maggirwar SB, Dewhurst S, et al. Akt inhibitors as an HIV-1 infected macrophage-specific antiviral therapy. Retrovirology. 2008; 5:11.

2). Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, et al. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007; 30:605–13.

3). Lucas A, Kim Y, Rivera-Pabon O, Chae S, Kim DH, Kim B. Targeting the PI3K/Akt cell survival pathway to induce cell death of HIV-1 infected macrophages with alkylphospholipid compounds. PLoS One. 2010; 5.

4). Chugh P, Fan S, Planelles V, Maggirwar SB, Dewhurst S, Kim B. Infection of human immunodeficiency virus and intracellular viral Tat protein exert a pro-survival effect in a human microglial cell line. J Mol Biol. 2007; 366:67–81.

5). Kim Y, Hollenbaugh JA, Kim DH, Kim B. Novel PI3K/Akt inhibitors screened by the cytoprotective function of human immunodeficiency virus type 1 Tat. PLoS One. 2011; 6:e21781.

6). Brown A, Zhang H, Lopez P, Pardo CA, Gartner S. In vitro modeling of the HIV-macrophage reservoir. J Leukoc Biol. 2006; 80:1127–35.
7). Schrier RD, McCutchan JA, Venable JC, Nelson JA, Wiley CA. T-cell-induced expression of human immunodeficiency virus in macrophages. J Virol. 1990; 64:3280–8.

8). Cosenza MA, Zhao ML, Lee SC. HIV-1 expression protects macrophages and microglia from apoptotic death. Neuropathol Appl Neurobiol. 2004; 30:478–90.

9). Irish BP, Khan ZK, Jain P, Nonnemacher MR, Pirrone V, Rahman S, et al. Molecular Mechanisms of Neurodegenerative Diseases Induced by Human Retroviruses: A Review. Am J Infect Dis. 2009; 5:231–58.

10). Jeong JJ, Kim DH. 5, 7-Dihydroxy-6-Methoxy-Flavonoids Eliminate HIV-1 D3-transfected Cytoprotective Macrophages by Inhibiting the PI3K/Akt Signaling Pathway. Phytother Res. 2015.
11). Jeong JJ, Kim B, Kim DH. Ginsenoside Rb1 eliminates HIV-1 (D3)-transduced cytoprotective human macrophages by inhibiting the AKT pathway. J Med Food. 2014; 17:849–54.

12). Suzuki T, Kobayashi M, Isatsu K, Nishihara T, Aiuchi T, Nakaya K, et al. Mechanisms involved in apoptosis of human macrophages induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans in the presence of cycloheximide. Infect Immun. 2004; 72:1856–65.
13). Aquaro S, Bagnarelli P, Guenci T, De Luca A, Clementi M, Balestra E, et al. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J Med Virol. 2002; 68:479–88.

14). Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005; 12(Suppl 1):893–904.

15). Kroes BH, van den Berg AJ, Quarles van Ufford HC, van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med. 1992; 58:499–504.

16). Hsieh TJ, Liu TZ, Chia YC, Chern CL, Lu FJ, Chuang MC, et al. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem Toxicol. 2004; 42:843–50.

17). Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010; 7:30.

18). Kim TW, Paveen S, Lee YH, Lee YS. Comparison of Cytotoxic Effects of Pentagalloylglucose, Gallic Acid, and its Derivatives Against Human Cancer MCF-7 and MDA MB-231 Cells. Bull Korean Chem Soc. 2014; 35:987–8.

19). Ahn MJ, Kim CY, Lee JS, Kim TG, Kim SH, Lee CK, et al. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002; 68:457–9.

20). Rivero-Buceta E, Carrero P, Doyaguez EG, Madrona A, Quesada E, Camarasa MJ, et al. Linear and branched alkyl-esters and amides of gallic acid and other (mono-, di- and tri-) hydroxy benzoyl derivatives as promising anti-HCV inhibitors. Eur J Med Chem. 2015; 92:656–71.

21). Kratz JM, Andrighetti-Frohner CR, Kolling DJ, Leal PC, Cirne-Santos CC, Yunes RA, et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem Inst Oswaldo Cruz. 2008; 103:437–42.

22). Modi M, Goel T, Das T, Malik S, Suri S, Rawat AK, et al. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian J Med Res. 2013; 137:540–8.
Go to : 
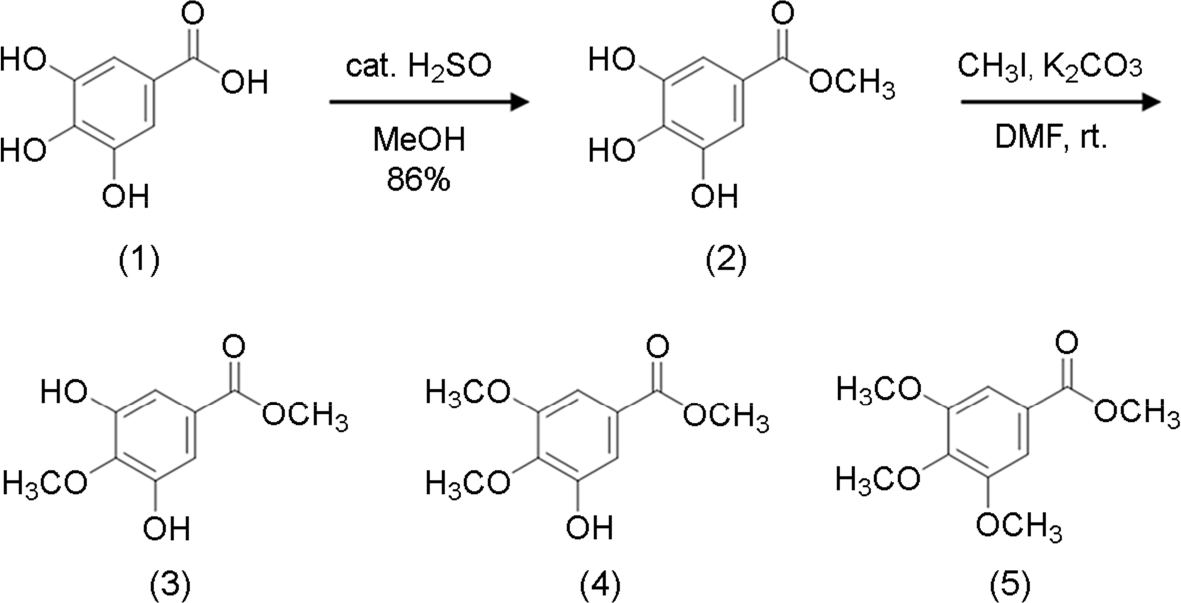 | Figure 1.Synthesis of gallic acid and its derivatives. (Compound 1), gallic acid; (compound 2), methyl gallate; (compound 3), methyl 4-O-methyl gallate; (compound 4), methyl 3,4-O-dimethyl gallate; (compound 5), methyl 3,4,5-O-trimethyl gallate. cat., catalytic; DMF, dimethylformamide; rt, room temperature. |
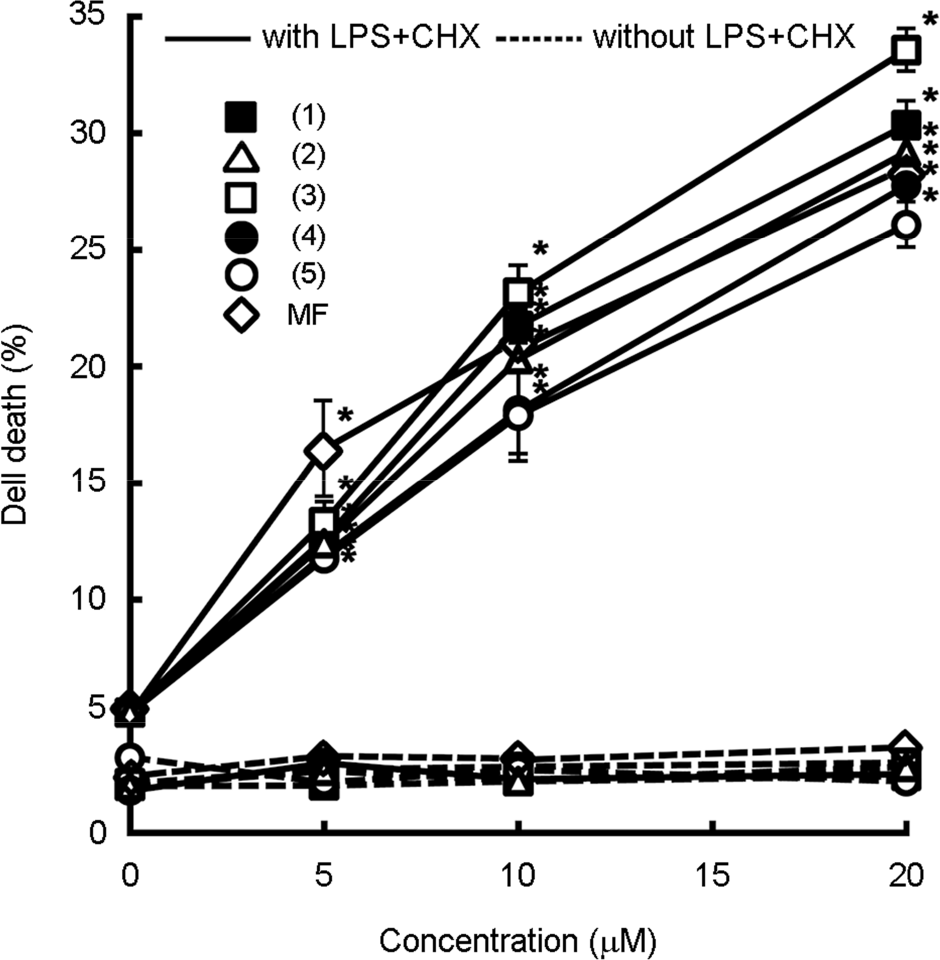 | Figure 2.The cytotoxic effects of gallic acid derivatives against cytoprotective HIV-1 Tat-transfected CHME5 cells. HIV-1 Tat-transfected CHME5 cells were treated with test compounds (0, 5, 10, and 20 μM) or miltefosine (MF, 20 μM) for 48 h. (Compound 1), gallic acid; (compound 2), methyl gallate; (compound 3), methyl 4-O-methyl gallate; (compound 4), methyl 3,4-O-dimethyl gallate; (compound 5), methyl 3,4,5-O-trimethyl gallate in the absence or presence of LPS/CHX. Cell death was determined by the trypan blue staining assay. All values are mean ± S.D. (n = 4). ∗, p <0.05 compared with 0 μM treatment group. |
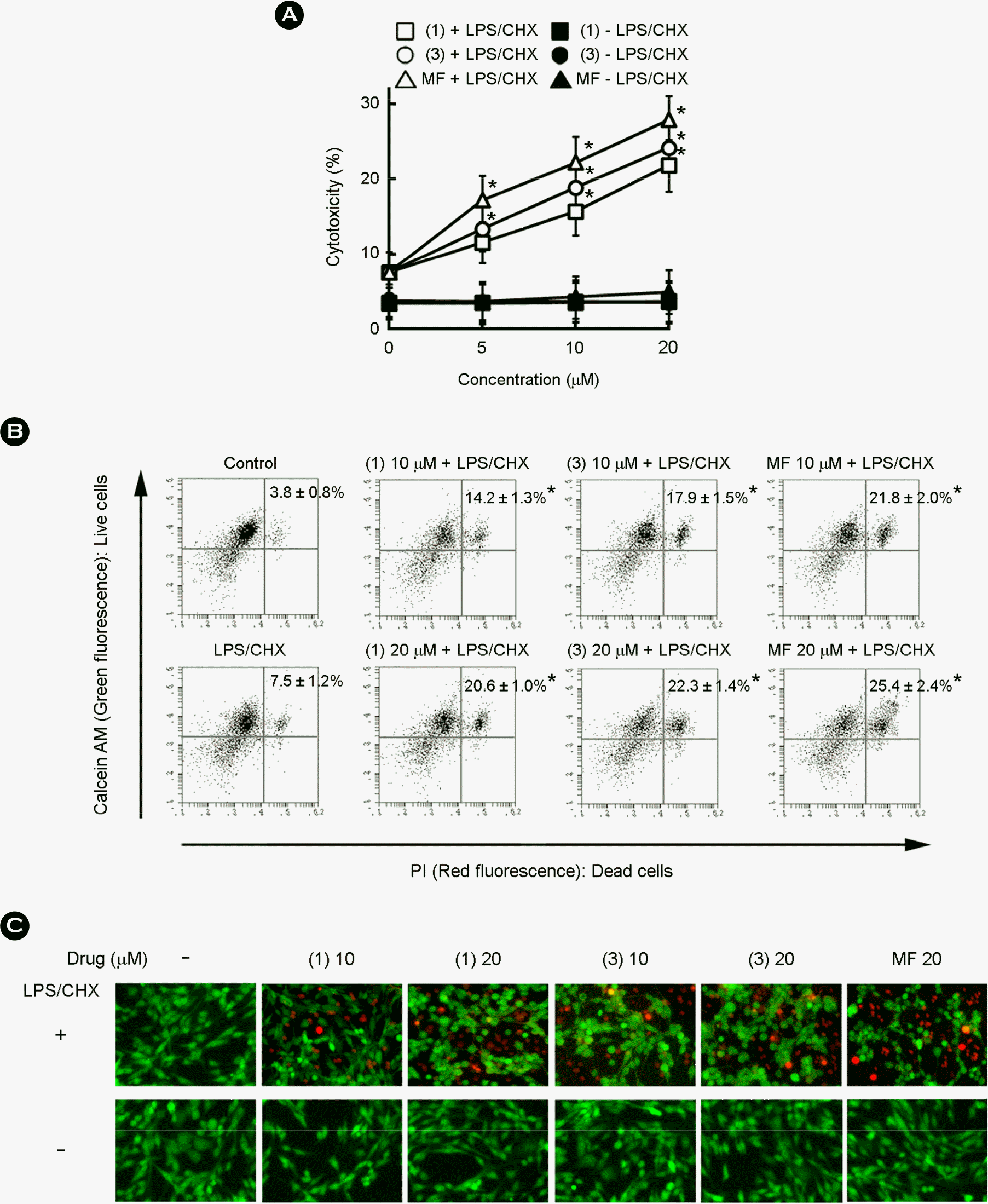 | Figure 3.The cytotoxic effects of gallic acid and methyl 4-O-methyl gallate against cytoprotective HIV-1 Tat-transfected CHME5 cells. (A) Cytotoxic effects of gallic acid (compound 1) and 4-methoxy methyl gallate (compound 3) were determined using trypan blue staining assay. (B) Cytotoxic effects of gallic acid (compound 1) and 4-methoxy methyl gallate (compound 3) by the calcein AM/PI using a flow cytometer (C6 Flow Cytometer® System) and a fluorescence microscope. HIV-1 Tat-transfected CHME5 cells were treated with LPS/CHX in the absence or presence of test compounds (0, 10, and 20 μM) or miltefosine (MF, 20 μM) for 48 h. Trypsinized cells stained with calcein AM/PI were measured by a flow cytometer. All values are the mean ± S.D. (n = 4). (C) Cytotoxicity of test compounds was measured using a fluorescence microscope. Cells were stained with calcein AM/PI to distinguish between dead (red) and live (green) cells. Images (merged red and green fields) are representatives of 3 experiments conducted in duplicate. ∗, p < 0.05 compared with LPS/CHX treatment group. |
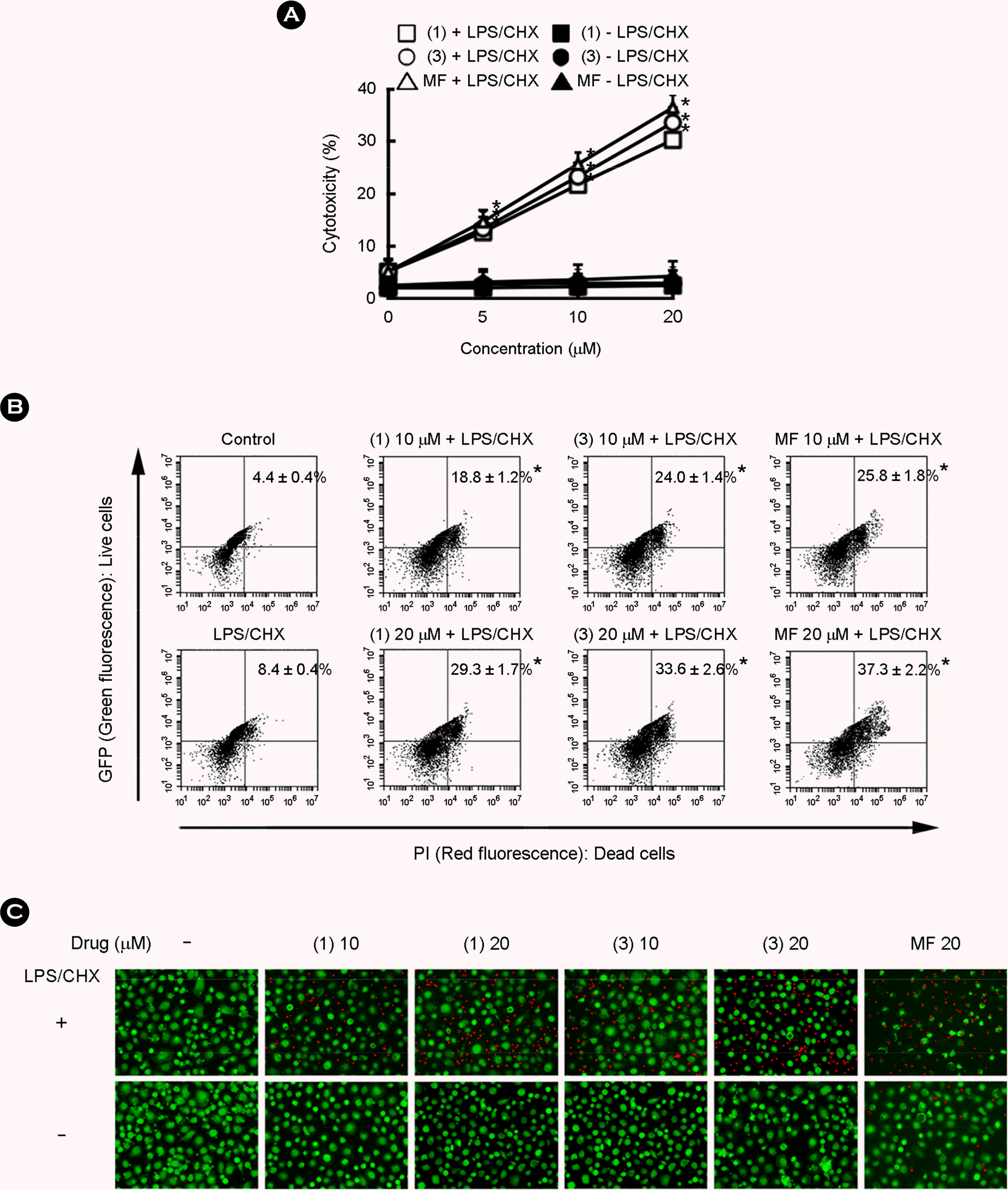 | Figure 4.Cytotoxic effects of gallic acid and methyl 4-O-methyl gallate against HIV-1-D3-infected human primary macrophages. (A) Cytotoxic effects of gallic acid (compound 1) and 4-methoxy methyl gallate (compound 3) were determined using trypan blue staining assay. (B) HIV-1-D3-infected primary macrophages were treated with LPS/CHX in the absence or presence of gallic acid (compound 1) or methyl 4-O-methyl gallate (compound 3) (0, 5, 10, and 20 μM). Cytotoxic effects of gallic acid (compound 1) and methyl 4-O-methyl gallate (compound 3) by PI/FACS assay. Cells stained with PI were examined by a flow cytometer. All values are mean ± S.D. (n = 4). (C) Cytotoxicity of compounds was determined using a fluorescence microscope. Cells were stained with PI to distinguish between dead (red) and live (green) cells. Images (merged red and green fields) are representatives of 3 experiments conducted in duplicate. ∗, p < 0.05 compared with LPS/CHX treatment group. |
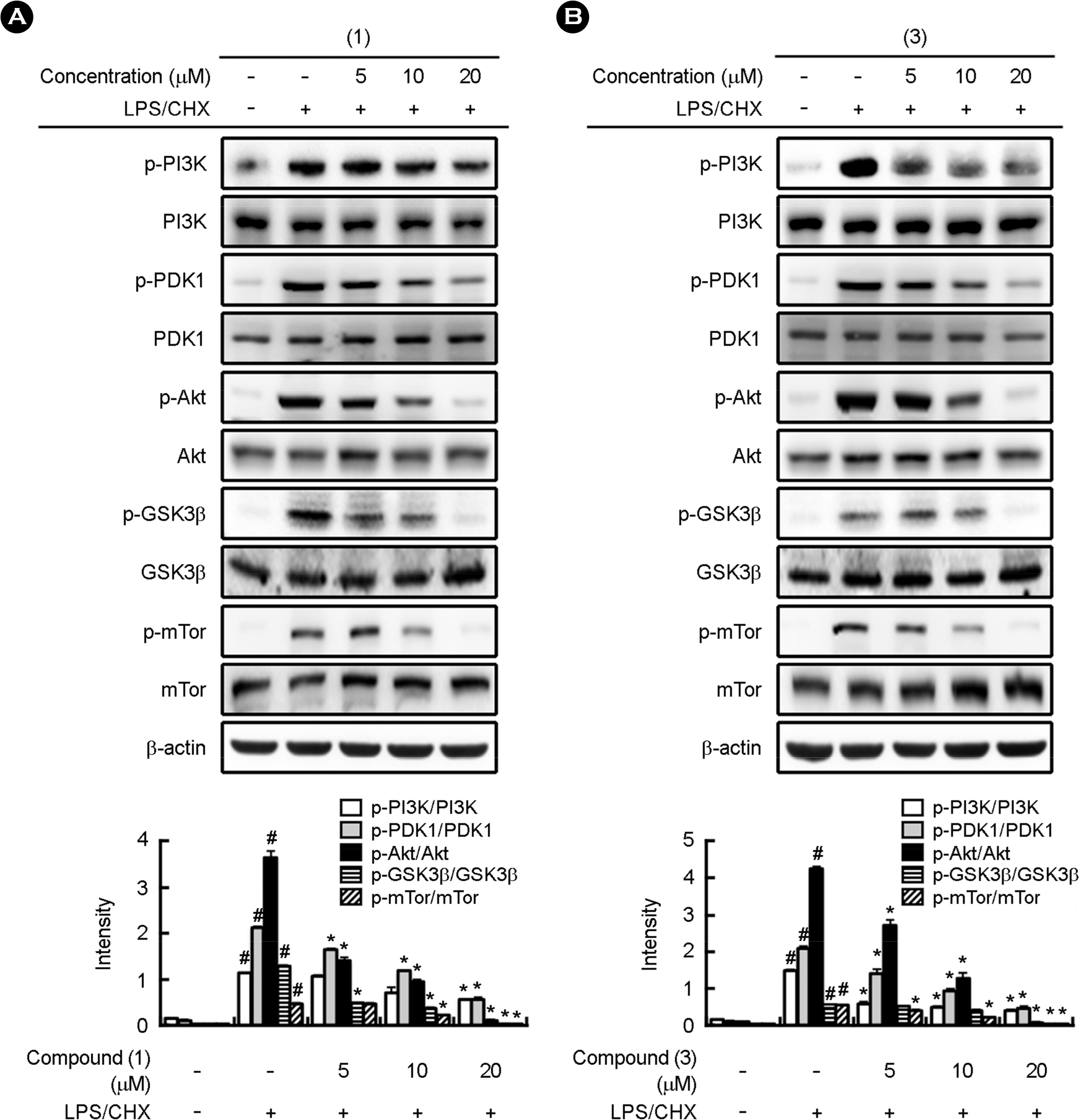 | Figure 5.Effects of gallic acid and 4-methoxy methyl gallate on the phosphorylation of PI3K, PDK1, Akt, GSK3β, mTOR and β-actin in LPS/CHX-stimulated HIV-1-D3-infected human primary macrophages. (A) Effects of gallic acid (compound 1). (B) Effect of methyl 4-O-methyl gallate (compound 3). HIV-1-D3-transfected primary macrophages were treated with LPS/CHX in the absence or presence of gallic acid (compound 1), methyl 4-O-methyl gallate (compound 3) (0, 5, 10, and 20 μM) for 120 min. Proteins were measured by immunoblotting. #, p < 0.05 compared with normal control; ∗, p < 0.05 compared with LPS/CHX treatment group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download